|
Induction Period
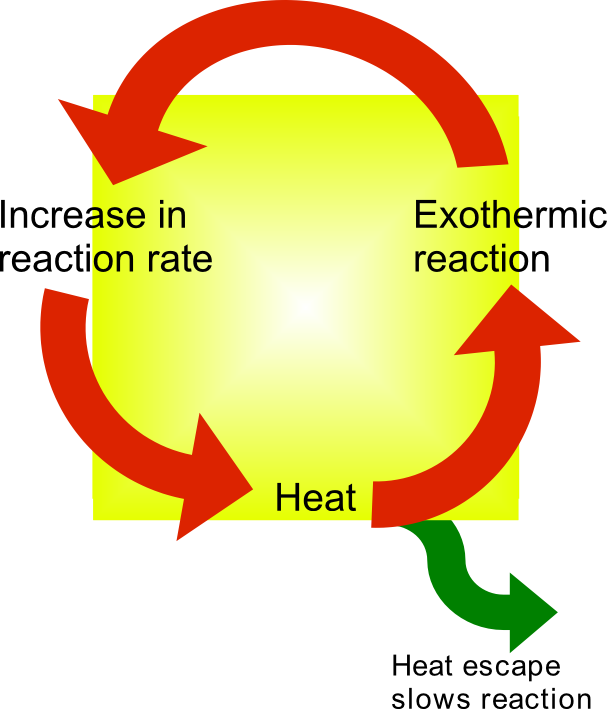
An induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions. In some catalytic reactions, a pre-catalyst needs to undergo a transformation to form the active catalyst, before the catalyst can take effect. Time is required for this transformation, hence the induction period. For example, with Wilkinson's catalyst, one triphenylphosphine ligand must dissociate to give the coordinatively unsaturated 14-electron species which can participate in the catalytic cycle: : Similarly, for an autocatalytic reaction, where one of the reaction products catalyzes the reaction itself, the rate of reaction is low initially until sufficient products have formed to catalyze the reaction. Reactions generally accelerate when heat is applied. Where a reaction is exothermic, the rate of the reaction may initially be low. As the reaction proceeds, heat is generat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigmoid Curve For An Autocatalytical Reaction , similar shape, term sometimes used for sigmoids
{{disambiguation ...
Sigmoid means resembling the lower-case Greek letter sigma (uppercase Σ, lowercase σ, lowercase in word-final position ς) or the Latin letter S. Specific uses include: * Sigmoid function, a mathematical function * Sigmoid colon, part of the large intestine or colon * Sigmoid sinus, two structures that drain blood from the bottom of the brain * Sigmoid arteries, a pair/trio of arteries in the lower abdomen See also * Ogee An ogee ( ) is the name given to objects, elements, and curves—often seen in architecture and building trades—that have been variously described as serpentine-, extended S-, or sigmoid-shaped. Ogees consist of a "double curve", the combinatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 35P. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Runaway Reaction
Thermal runaway describes a process that is accelerated by increased temperature, in turn releasing energy that further increases temperature. Thermal runaway occurs in situations where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result. It is a kind of uncontrolled positive feedback. In chemistry (and chemical engineering), thermal runaway is associated with strongly exothermic reactions that are accelerated by temperature rise. In electrical engineering, thermal runaway is typically associated with increased current flow and power dissipation. Thermal runaway can occur in civil engineering, notably when the heat released by large amounts of curing concrete is not controlled. In astrophysics, runaway nuclear fusion reactions in stars can lead to nova and several types of supernova explosions, and also occur as a less dramatic event in the normal evolution of solar-mass stars, the "hel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson's Catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalytic and organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordinatively Unsaturated
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and analytical tests. For instance, IUPAC nomenclature is a system of naming conventions used to describe the type and loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autocatalytic Reaction
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1999) p.151-2 Such a reaction is called an autocatalytic reaction. A ''set'' of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set). Chemical reactions A chemical reaction of two reactants and two products can be written as : \alpha A + \beta B \rightleftharpoons \sigma S + \tau T where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). The term ''exothermic'' was first coined by 19th-century French chemist Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy usually in the form of heat. The concept is frequently applied in the physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Two types of chemical reactions Exothermic and endothermic describe two types of chemical reactions or systems found in nature, as follows: Exothermic After an exothermic reaction, more energy has been released to the surroundings than was absorbed to initiate and maintain the reaction. An example would be the burn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Runaway
Thermal runaway describes a process that is accelerated by increased temperature, in turn releasing energy that further increases temperature. Thermal runaway occurs in situations where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result. It is a kind of uncontrolled positive feedback. In chemistry (and chemical engineering), thermal runaway is associated with strongly exothermic reactions that are accelerated by temperature rise. In electrical engineering, thermal runaway is typically associated with increased current flow and power dissipation. Thermal runaway can occur in civil engineering, notably when the heat released by large amounts of curing concrete is not controlled. In astrophysics, runaway nuclear fusion reactions in stars can lead to nova and several types of supernova explosions, and also occur as a less dramatic event in the normal evolution of solar-mass stars, the " he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |