|
Isothermal
An isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi-equilibrium). In contrast, an ''adiabatic process'' is where a system exchanges no heat with its surroundings (''Q'' = 0). Simply, we can say that in an isothermal process * T = \text * \Delta T = 0 * dT = 0 * For ideal gases only, internal energy \Delta U = 0 while in adiabatic processes: * Q = 0. Etymology The noun '' isotherm'' is derived from the Ancient Greek words (), meaning "equal", and (), meaning "heat". Examples Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isothermal Process
An isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi-equilibrium). In contrast, an '' adiabatic process'' is where a system exchanges no heat with its surroundings (''Q'' = 0). Simply, we can say that in an isothermal process * T = \text * \Delta T = 0 * dT = 0 * For ideal gases only, internal energy \Delta U = 0 while in adiabatic processes: * Q = 0. Etymology The noun '' isotherm'' is derived from the Ancient Greek words (), meaning "equal", and (), meaning "heat". Examples Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem (thermodynamics), Carnot's theorem, it provides an upper limit on the Thermal efficiency, efficiency of any classical Heat engine, thermodynamic engine during the conversion of heat into Work (thermodynamics), work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a Thermodynamic system, system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is Reversible process (thermodynamics), reversible, and entropy is Conserved quantity, conserved, merely transferred between the th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
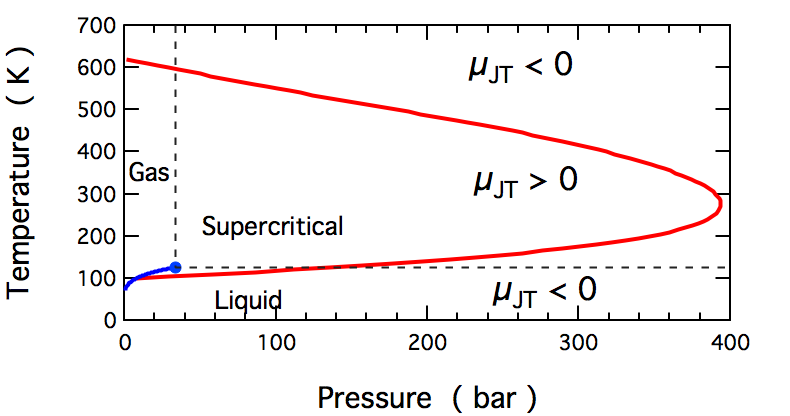
Joule–Thomson Effect
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a Real gas, ''real'' gas or liquid (as differentiated from an ideal gas) when it is expanding; typically caused by the pressure loss from flow through a expansion valve (steam engine), valve or enthalpy#Throttling, porous plug while keeping it insulated so that Adiabatic process, no heat is exchanged with the environment. This procedure is called a ''throttling process'' or ''Joule–Thomson process''. The effect is purely due to deviation from ideality, as any ideal gas has no JT effect. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases rise in temperature when forced through a porous plug at room temperature, but lowers in temperature when already at lower temperatures. Most liquids such as Hydraulics, hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Process (thermodynamics)
In thermodynamics, a reversible process is a process, involving a system and its surroundings, whose direction can be reversed by infinitesimal changes in some properties of the surroundings, such as pressure or temperature. Throughout an entire reversible process, the system is in thermodynamic equilibrium, both physical and chemical, and ''nearly'' in pressure and temperature equilibrium with its surroundings. This prevents unbalanced forces and acceleration of moving system boundaries, which in turn avoids friction and other dissipation. To maintain equilibrium, reversible processes are extremely slow ( ''quasistatic''). The process must occur slowly enough that after some small change in a thermodynamic parameter, the physical processes in the system have enough time for the other parameters to self-adjust to match the new, changed parameter value. For example, if a container of water has sat in a room long enough to match the steady temperature of the surrounding air, for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Engine
A heat engine is a system that transfers thermal energy to do mechanical or electrical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
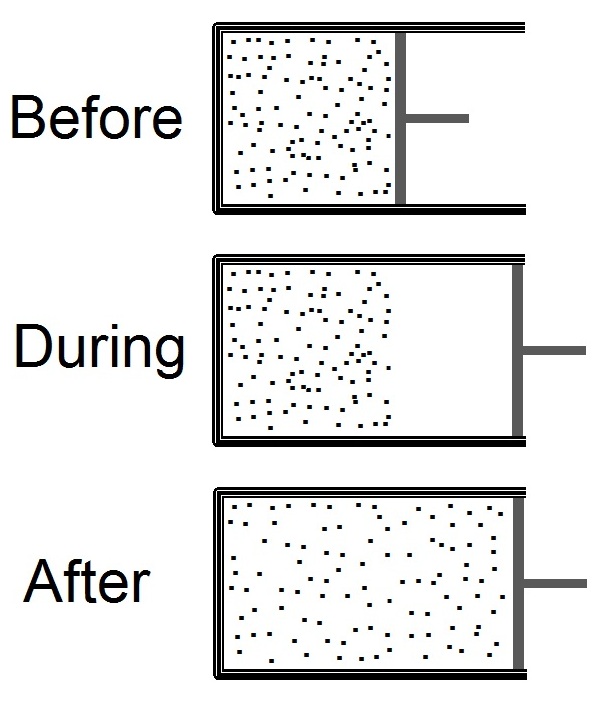
Joule Expansion
The Joule expansion (a subset of free expansion) is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container (via a small partition), with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container. The Joule expansion, treated as a thought experiment involving ideal gases, is a useful exercise in classical thermodynamics. It provides a convenient example for calculating changes in thermodynamic quantities, including the resulting increase in entropy of the universe (entropy production) that results from this inherently irreversible process. An actual Joule expansion experiment necessarily involves real gases; the temperature change in such a process provides a measure of intermolecular forces. This type of expansion is named after James Prescott Joule who used this expansion, in 1845, in his study for the mechanical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process
An adiabatic process (''adiabatic'' ) is a type of thermodynamic process that occurs without transferring heat between the thermodynamic system and its Environment (systems), environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as Work (thermodynamics), work and/or mass flow.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. The opposite term to "adiabatic" is ''diabatic''. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of fire, flame temperature by assuming combustion loses no heat to its surroundings. In meteorology, adiabatic expansion an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Process
Classical thermodynamics considers three main kinds of thermodynamic processes: (1) changes in a system, (2) cycles in a system, and (3) flow processes. (1) A Thermodynamic process is a process in which the thermodynamic state of a system is changed. A change in a system is defined by a passage from an initial to a final state of thermodynamic equilibrium. In classical thermodynamics, the actual course of the process is not the primary concern, and often is ignored. A state of thermodynamic equilibrium endures unchangingly unless it is interrupted by a thermodynamic operation that initiates a thermodynamic process. The equilibrium states are each respectively fully specified by a suitable set of thermodynamic state variables, that depend only on the current state of the system, not on the path taken by the processes that produce the state. In general, during the actual course of a thermodynamic process, the system may pass through physical states which are not describable as th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the ''spontaneity'' of processes. The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics. History In 1865, the German physicist Rudolf Clausius, in his ''Mechanical Theory of Heat'', suggested that the principles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accounting for the gains and losses of energy due to changes in its internal state, including such quantities as magnetization. It excludes the kinetic energy of motion of the system as a whole and the potential energy of position of the system as a whole, with respect to its surroundings and external force fields. It includes the thermal energy, ''i.e.'', the constituent particles' kinetic energies of motion relative to the motion of the system as a whole. Without a thermodynamic process, the internal energy of an isolated system cannot change, as expressed in the law of conservation of energy, a foundation of the first law of thermodynamics. The notion has been introduced to describe the systems characterized by temperature variations, te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular Forces
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles (e.g. atoms or ions). Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The first reference to the nature of microscopic forces is found in Alexis Clairaut's work ''Théorie de la figure de la Terre,'' published in Paris in 1743. Other scientists who have contributed to the investigation of microscopic forces include: Laplace, Gauss, Maxwell, Boltzmann and Pauling. Attractive intermolecular forces are categorized into the following type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Watt
James Watt (; 30 January 1736 (19 January 1736 OS) – 25 August 1819) was a Scottish inventor, mechanical engineer, and chemist who improved on Thomas Newcomen's 1712 Newcomen steam engine with his Watt steam engine in 1776, which was fundamental to the changes brought by the Industrial Revolution in both his native Great Britain and the rest of the world. While working as an instrument maker at the University of Glasgow, Watt became interested in the technology of steam engines. At the time engineers such as John Smeaton were aware of the inefficiencies of Newcomen's engine and aimed to improve it. Watt's insight was to realise that contemporary engine designs wasted a great deal of energy by repeatedly cooling and reheating the cylinder. Watt introduced a design enhancement, the separate condenser, which avoided this waste of energy and radically improved the power, efficiency, and cost-effectiveness of steam engines. Eventually, he adapted his engine to produce rot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |