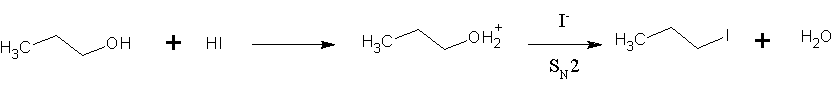|
Isopropyl Iodide
Isopropyl iodide is the organoiodine compound with the formula (CH3)2CHI. It is colorless, flammable, and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of iodine. Preparation Isopropyl iodide is prepared by iodination of isopropyl alcohol using hydrogen iodide or, equivalently, with a mixture of glycerol, iodine, and phosphorus.Merck Index of Chemicals and Drugs, 9th ed., monograph 5074 An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of sodium iodide (Finkelstein reaction The Finkelstein reaction named after the German chemist Hans Finkelstein, is an SN2 reaction (Substitution Nucleophilic Bimolecular reaction) that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the react ...):Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1989 :(CH3)2CHBr + NaI → (CH3)2CHI + NaBr References {{Organohalide-st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile (ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoalkanes
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt. Structure, bonding, general properties Almost all organoiodine compounds feature iodide connected to one carbon center. These are usually classified as derivatives of I−. Some organoiodine compounds feature iodine in higher oxidation states. The C–I bond is the weakest of the carbon–halogen bonds. These bond strengths correlate with the electronegativity of the halogen, decreasing in the order F > Cl > Br > I. This periodic order also follows the atomic radius of halogens and the length of the carbon-halogen bond. For example, in the molecules represented by CH3X, where X is a halide, the carbon-X bonds have strengths, or bond dissociation energies, of 115, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Finkelstein Reaction
The Finkelstein reaction named after the German chemist Hans Finkelstein, is an SN2 reaction (Substitution Nucleophilic Bimolecular reaction) that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of halide salts, or by using a large excess of the halide salt. :R–X + X′− R–X′ + X− Method The classic Finkelstein reaction entails the conversion of an alkyl chloride or an alkyl bromide to an alkyl iodide by treatment with a solution of sodium iodide in acetone. Sodium iodide is soluble in acetone while sodium chloride and sodium bromide are not. The reaction is driven toward products by mass action due to the precipitation of the poorly soluble NaCl or NaBr. An example involves the conversion of the ethyl ester of 5-bromovaleric acid to the iodide: :EtO2C(CH2)4Br + NaI → EtO2C(CH2)4I + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt. Uses Food supplement Sodium iodide, as well as potassium iodide, is commonly used to treat and prevent iodine deficiency. Iodized table salt contains 10 ppm iodide. Organic synthesis Sodium iodide is used for conversion of alkyl chlorides into alkyl iodides. This method, the Finkelstein reaction, relies on the insolubility of sodium chloride in acetone to drive the reaction: ::R–Cl + NaI → R–I + NaCl Nuclear medicine Some radioactive iodide salts of sodium, including Na 125I and Na 131I, have radioph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-propyl Bromide
2-Bromopropane, also known as isopropyl bromide and 2-propyl bromide, is the halogenated hydrocarbon with the formula CH3CHBrCH3. It is a colorless liquid. It is used for introducing the isopropyl functional group in organic synthesis. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid. Preparation 2-Bromopropane is commercially available. It may be prepared in the ordinary manner of alkyl bromides, by reacting isopropanol with phosphorus and bromine, or with phosphorus tribromide. Safety Short-chain alkyl halides are often carcinogenic. The bromine atom is at the secondary position, which allows the molecule to undergo dehydrohalogenation easily to give propene, which escapes as a gas and can rupture closed reaction vessels. When this reagent is used in base catalyzed reactions, potassium carbonate should be used in place of sodium or potassium hydroxide. Further reading *Max Gergel Max G. Gergel (July 24, 1921 - July 5, 2017) was an American chemist and ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the " Morning Star", the planet Venus. The term '' phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is not pure hydrogen iodide, but a mixture containing it. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by mass. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flammable
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable material catches fire immediately on exposure to flame. The degree of flammability or combustibility in air depends largely upon the volatility of the material - this is related to its composition-specific vapour pressure, which is temperature dependent. The quantity of vapour produced can be enhanced by increasing the surface area of the material forming a mist or dust. Take wood as an example. Finely divided wood dust can undergo explosive combustion and produce a blast wave. A piece of paper (made from wood) catches on fire quite easily. A heavy oak desk is much harder to ignite, even though the wood fibre is the same in all three materials. Common sense (and indeed scientific consensus until the mid-1700s) would seem to suggest that ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Iodide
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th ed., monograph 3753 On contact with air, especially on the effect of light, it decomposes and turns yellow or reddish from dissolved iodine. It may also be prepared by reaction between hydroiodic acid and ethanol distilling off the ethyl iodide. Ethyl iodide should be stored in the presence of copper powder to avoid rapid decomposition, though even with this method samples do not last more than 1 year. Because iodide is a good leaving group, ethyl iodide is an excellent ethylating agent. It is also used as the hydrogen radical promoter. Production Ethyl iodide is prepared by using red phosphorus, absolute ethanol and iodine. The iodine dissolves in the ethanol, where it reacts with the solid phosphorus to form phosphorus triiodide Phosphorus triiodide (PI3) is an i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |