|
Iron Peak
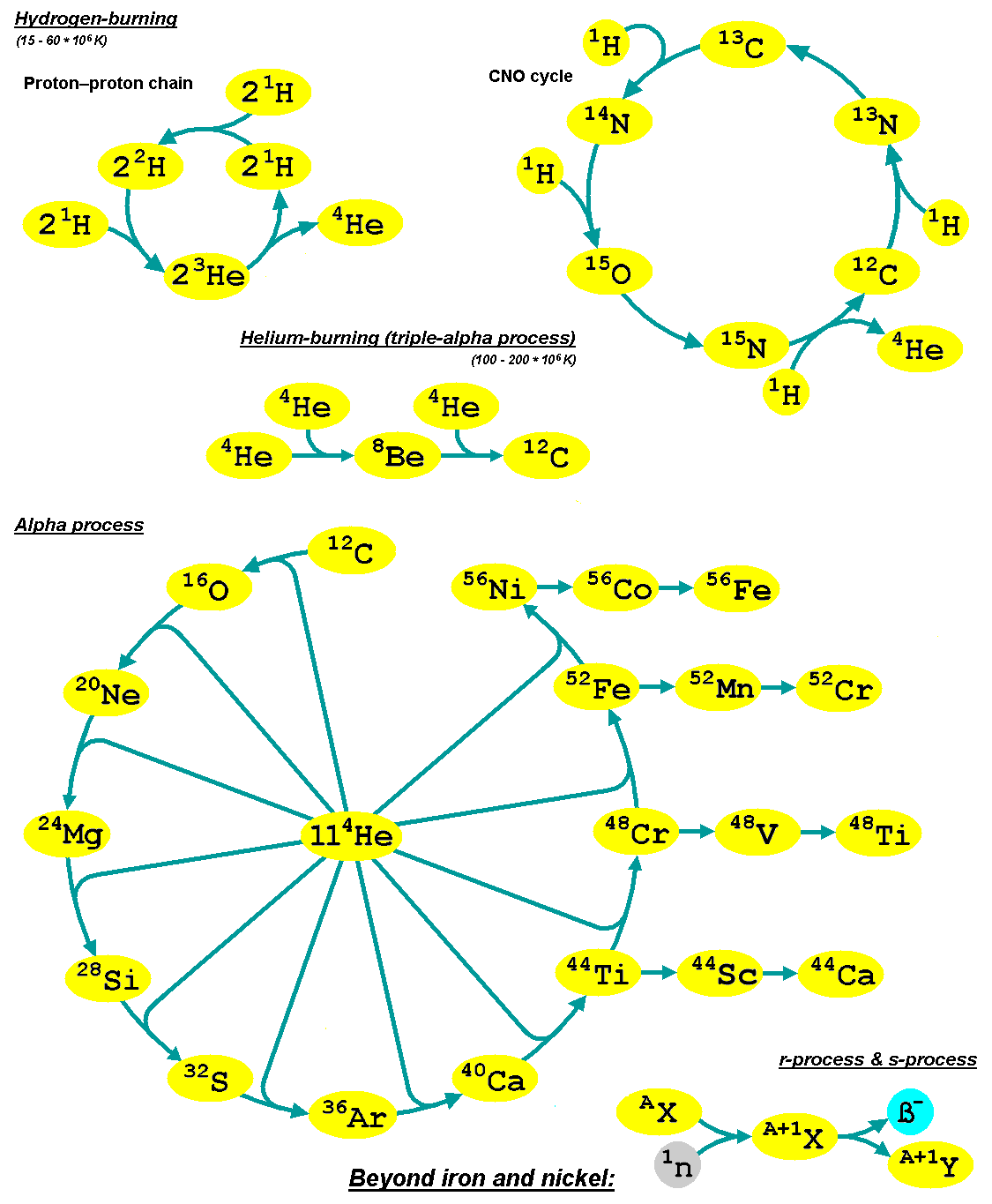
The iron peak is a local maximum in the vicinity of Fe ( Cr, Mn, Fe, Co and Ni) on the graph of the abundances of the chemical elements. For elements lighter than iron on the periodic table, nuclear fusion releases energy. For iron, and for all of the heavier elements, nuclear fusion consumes energy. Chemical elements up to the iron peak are produced in ordinary stellar nucleosynthesis, with the alpha elements being particularly abundant. Some heavier elements are produced by less efficient processes such as the r-process and s-process. Elements with atomic numbers close to iron are produced in large quantities in supernova due to explosive oxygen and silicon fusion, followed by radioactive decay of nuclei such as Nickel-56. On average, heavier elements are less abundant in the universe, but some of those near iron are comparatively more abundant than would be expected from this trend. Binding energy A graph of the nuclear binding energy per nucleon for all the eleme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Maximum
In mathematical analysis, the maxima and minima (the respective plurals of maximum and minimum) of a function, known collectively as extrema (the plural of extremum), are the largest and smallest value of the function, either within a given range (the ''local'' or ''relative'' extrema), or on the entire domain (the ''global'' or ''absolute'' extrema). Pierre de Fermat was one of the first mathematicians to propose a general technique, adequality, for finding the maxima and minima of functions. As defined in set theory, the maximum and minimum of a set are the greatest and least elements in the set, respectively. Unbounded infinite sets, such as the set of real numbers, have no minimum or maximum. Definition A real-valued function ''f'' defined on a domain ''X'' has a global (or absolute) maximum point at ''x''∗, if for all ''x'' in ''X''. Similarly, the function has a global (or absolute) minimum point at ''x''∗, if for all ''x'' in ''X''. The value of the function at a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximately half the atomic nuclei heavier than iron. In the ''s''-process, a seed nucleus undergoes neutron capture to form an isotope with one higher atomic mass. If the new isotope is stable, a series of increases in mass can occur, but if it is unstable, then beta decay will occur, producing an element of the next higher atomic number. The process is ''slow'' (hence the name) in the sense that there is sufficient time for this radioactive decay to occur before another neutron is captured. A series of these reactions produces stable isotopes by moving along the valley of beta-decay stable isobars in the table of nuclides. A range of elements and isotopes can be produced by the ''s''-process, because of the intervention of alpha decay steps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process. The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, further reactions below take place, listed below. Each step only consumes helium and the product of the previous reaction. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \end The energy produced each the reaction, , is primarily in the gamma ray (), with a small amount taken by the byproduct element, as added momentum. It is a common misconception that the above sequence ends at \, _^\mathrm \, (or \, _^\mathrm \,, which is a decay product of \, _^\mathrm \,) because it is the most tightly bound nuclide - i.e., having the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons. It is a stable isotope, with the highest binding energy per nucleon of any known nuclide (8.7945 MeV). It is often stated that 56Fe is the "most stable nucleus", but only because 56Fe has the lowest ''mass'' per nucleon (not binding energy per nucleon) of all nuclides. The lower mass per nucleon of 56Fe is possible because 56Fe has 26/56 ≈ 46.43% protons, while 62Ni has only 28/62 ≈ 45.16% protons; and the larger fraction of lighter protons in 56Fe lowers its mean mass-per-nucleon ratio in a way that has no effect on its binding energy. Properties The high binding energy of nickel isotopes in general makes nickel an "end product" of many nuclear reactions (including neutron capture reactions) throughout the universe and accounts for the high relative abundance of nickel—although most of the nickel in space (and thus produced by supernova explosions) is nickel-58 (the most common isotope) and nickel- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-58
Naturally occurring iron (26Fe) consists of four stable isotopes: 5.845% of 54Fe (possibly radioactive with a half-life over years), 91.754% of 56Fe, 2.119% of 57Fe and 0.286% of 58Fe. There are 24 known radioactive isotopes, the most stable of which are 60Fe (half-life 2.6 million years) and 55Fe (half-life 2.7 years). Much of the past work on measuring the isotopic composition of Fe has centered on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometry technology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, although applications to biological and industrial systems are beginning to emerge. List of isotopes , - , rowspan=2, 45Fe , rowspan=2 style="text-align:right" , 26 , rowspan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sky, night, but their immense distances from Earth make them appear as fixed stars, fixed points of light. The most prominent stars have been categorised into constellations and asterism (astronomy), asterisms, and many of the brightest stars have proper names. Astronomers have assembled star catalogues that identify the known stars and provide standardized stellar designations. The observable universe contains an estimated to stars. Only about 4,000 of these stars are visible to the naked eye, all within the Milky Way galaxy. A star's life star formation, begins with the gravitational collapse of a gaseous nebula of material composed primarily of hydrogen, along with helium and trace amounts of heavier elements. Its stellar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission of heavy elements was discovered on Monday 19 December 1938, by German chemist Otto Hahn and his assistant Fritz Strassmann in cooperation with Austrian-Swedish physicist Lise Meitner. Hahn understood that a "burst" of the atomic nuclei had occurred. Meitner explained it theoretically in January 1939 along with her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). Like nuclear fusion, for fission to produce energy, the total binding energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spontaneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number). Until the 1960s, nucleons were thought to be elementary particles, not made up of smaller parts. Now they are known to be composite particles, made of three quarks bound together by the strong interaction. The interaction between two or more nucleons is called internucleon interaction or nuclear force, which is also ultimately caused by the strong interaction. (Before the discovery of quarks, the term "strong interaction" referred to just internucleon interactions.) Nucleons sit at the boundary where particle physics and nuclear physics overlap. Particle physics, particularly quantum chromodynamics, provides the fundamental equations that describe the properties of quarks and of the strong interaction. These equations describe quantitatively how quarks can bind toget ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Energy Curve - Common Isotopes
Binding may refer to: Computing * Binding, associating a network socket with a local port number and IP address * Data binding, the technique of connecting two data elements together ** UI data binding, linking a user interface element to an element of a domain model, such as a database field ** XML data binding, representing XML document data using objects and classes * Key binding, or keyboard shortcut, mapping key combinations to software functionality * Language binding, a library providing a functional interface to second library in a different programming language * Name binding, the association of code or data with an identifier in a programming language ** Late binding, name binding which is resolved at run-time rather than in pre-execution time Science * Binding problem, a term for several problems in cognitive science and philosophy ** Neural binding, synchronous activity of neurons and neuronal ensembles * Molecular binding, an attractive interaction between two molecule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |