|
Iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56. Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei. Nickel-62, a relatively rare isotope of nickel, has a higher nuclear binding energy per nucleon; this is consistent with having a higher mass-per-nucleon because nickel-62 has a greater proportion of neutrons, which are slightly more massive than protons. (See the nickel-62 article for more). Light elements undergoing nuclear fusion and heavy elements undergoing nuclear fission release energy as their nucleons bind more tightly, so 62Ni might be expected to be common. However, during nucleosynthesis in stars the competition between photodisintegration and alpha capturing causes more 56Ni to be produced than 62Ni (56Fe is produced later in the star's ejection shell as 56Ni decays). Production of these elements has decreased consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Iron
Naturally occurring iron (26Fe) consists of four stable isotopes: 5.845% of 54Fe (possibly radioactive with a half-life over years), 91.754% of 56Fe, 2.119% of 57Fe and 0.286% of 58Fe. There are 24 known radioactive isotopes, the most stable of which are 60Fe (half-life 2.6 million years) and 55Fe (half-life 2.7 years). Much of the past work on measuring the isotopic composition of Fe has centered on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometry technology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, although applications to biological and industrial systems are beginning to emerge. List of isotopes , - , rowspan=2, 45Fe , rowspan=2 style="text-align:right" , 26 , rowspa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Binding Energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means. The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons. The difference in mass can be calculated by the Einstein equation, , where ''E'' is the nuclear bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Energy Curve - Common Isotopes
Binding may refer to: Computing * Binding, associating a network socket with a local port number and IP address * Data binding, the technique of connecting two data elements together ** UI data binding, linking a user interface element to an element of a domain model, such as a database field ** XML data binding, representing XML document data using objects and classes * Key binding, or keyboard shortcut, mapping key combinations to software functionality * Language binding, a library providing a functional interface to second library in a different programming language * Name binding, the association of code or data with an identifier in a programming language ** Late binding, name binding which is resolved at run-time rather than in pre-execution time Science * Binding problem, a term for several problems in cognitive science and philosophy ** Neural binding, synchronous activity of neurons and neuronal ensembles * Molecular binding, an attractive interaction between two molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
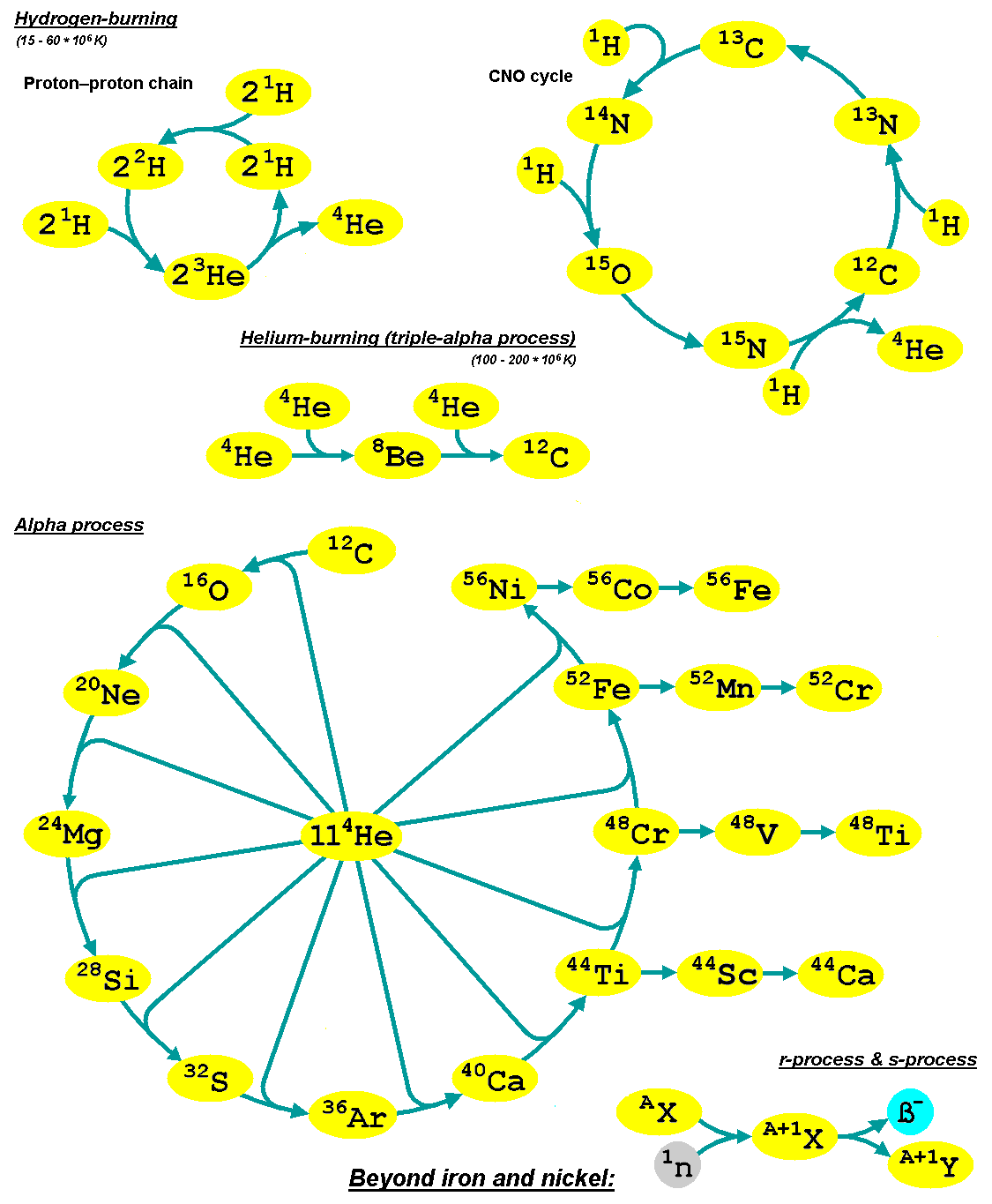
Alpha Process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process. The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, further reactions below take place, listed below. Each step only consumes helium and the product of the previous reaction. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \end The energy produced each the reaction, , is primarily in the gamma ray (), with a small amount taken by the byproduct element, as added momentum. It is a common misconception that the above sequence ends at \, _^\mathrm \, (or \, _^\mathrm \,, which is a decay product of \, _^\mathrm \,) because it is the most tightly bound nuclide - i.e., having the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese-56
Naturally occurring manganese (25Mn) is composed of one stable isotope, 55Mn. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.3 days, and 52Mn with a half-life of 5.591 days. All of the remaining radioactive isotopes have half-lives that are less than 3 hours and the majority of these have half-lives that are less than a minute, but only 45Mn has an unknown half-life. The least stable is 44Mn with a half-life shorter than 105 nanoseconds. This element also has 3 meta states. Manganese is part of the iron group of elements, which are thought to be synthesized in large stars shortly before supernova explosions. 53Mn decays to 53 Cr with a half-life of 3.7 million years. Because of its relatively short half-life, 53Mn occurs only in tiny amounts due to the action of cosmic rays on iron in rocks. Manganese isotopic contents are typically combined with chromium isotopic contents and have found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-55
Iron-55 (55Fe) is a radioactive isotope of iron with a nucleus containing 26 protons and 29 neutrons. It decays by electron capture to manganese-55 and this process has a half-life of 2.737 years. The emitted X-rays can be used as an X-ray source for various scientific analysis methods, such as X-ray diffraction. Iron-55 is also a source for Auger electrons, which are produced during the decay. Decay Iron-55 decays via electron capture to manganese-55 with a half-life of 2.737 years. The electrons around the nucleus rapidly adjust themselves to the lowered charge without leaving their shell, and shortly thereafter the vacancy in the "K" shell left by the nuclear-captured electron is filled by an electron from a higher shell. The difference in energy is released by emitting Auger electrons of 5.19 keV, with a probability of about 60%, K-alpha-1 X-rays with energy of 5.89875 keV and a probability about 16.2%, K-alpha-2 X-rays with energy of 5.88765 keV and a probability of about 8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Star
In astronomy, an iron star is a hypothetical type of compact star. Unrelatedly, the term "iron star" is also used for blue supergiants which have a forest of forbidden FeII lines in their spectra. They are potentially quiescent hot luminous blue variables. Eta Carinae has been described as a prototypical example. Formation An iron star is a hypothetical type of compact star that could occur in the universe in the extremely far future, after perhaps 101500 years. The premise behind the formation of iron stars states that cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into iron-56 nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting stellar-mass objects to cold spheres of iron. The formation of these stars is only a possibility if protons do not decay. Though the surface of a neutron star may be iron according to some predictions, it is distinct from an iron star. By the end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reviews Of Modern Physics
''Reviews of Modern Physics'' (abbreviated RMP) is a quarterly peer-reviewed scientific journal published by the American Physical Society. It was established in 1929 and the current editor-in-chief is Michael Thoennessen. The journal publishes review articles, usually by established researchers, on all aspects of physics Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which rel ... and related fields. The reviews are usually accessible to non-specialists and serve as introductory material to graduate students, which survey recent work, discuss key problems to be solved and provide perspectives toward the end. References External links * Publications established in 1929 Physics review journals Quarterly journals English-language journals American Physical Society academic jour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Future Of An Expanding Universe
Observations suggest that the expansion of the universe will continue forever. The prevailing theory is that the universe will cool as it expands, eventually becoming too cold to sustain life. For this reason, this future scenario once popularly called " Heat Death" is now known as the "Big Chill" or "Big Freeze". If dark energy—represented by the cosmological constant, a ''constant'' energy density filling space homogeneously, or scalar fields, such as quintessence or moduli, ''dynamic'' quantities whose energy density can vary in time and space—accelerates the expansion of the universe, then the space between clusters of galaxies will grow at an increasing rate. Redshift will stretch ancient, incoming photons (even gamma rays) to undetectably long wavelengths and low energies. Stars are expected to form normally for 1012 to 1014 (1–100 trillion) years, but eventually the supply of gas needed for star formation will be exhausted. As existing stars run out of fuel and cea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Star
In astronomy, an iron star is a hypothetical type of compact star. Unrelatedly, the term "iron star" is also used for blue supergiants which have a forest of forbidden FeII lines in their spectra. They are potentially quiescent hot luminous blue variables. Eta Carinae has been described as a prototypical example. Formation An iron star is a hypothetical type of compact star that could occur in the universe in the extremely far future, after perhaps 101500 years. The premise behind the formation of iron stars states that cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into iron-56 nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting stellar-mass objects to cold spheres of iron. The formation of these stars is only a possibility if protons do not decay. Though the surface of a neutron star may be iron according to some predictions, it is distinct from an iron star. By the end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the universe. According to this theory, space and time emerged together ago, and the universe has been expanding ever since the Big Bang. While the spatial size of the entire universe is unknown, it is possible to measure the size of the observable universe, which is approximately 93 billion light-years in diameter at the present day. Some of the earliest cosmological models of the universe were developed by ancient Greek and Indian philosophers and were geocentric, placing Earth at the center. Over the centuries, more precise astronomical observations led Nicolaus Copernicus to develop the heliocentric model with the Sun at the center of the Solar System. In developing the law of universal gravitation, Isaac Newton built upon Copernicus's work as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
