|
Ionic Partition Diagram
Similar to Pourbaix's diagram for the speciation of redox species as a function of the redox potential and the pH, ionic partition diagrams indicate in which an acid or a base are predominantly present in a biphasic system as a function of the Galvani potential difference between the two phases and the pH of the aqueous solution Ionic partition diagram of an hydrophilic acid AH in a biphasic water/organic solvent system. At a high aqueous pH, the acid is in the anionic form and can exist in both phases according to the Galvani potential difference. The Nernst equation for the distribution of the anion, ignoring the activity coefficients is written. Thus, the separation limit between the anionic form in water and the organic solvent () is a horizontal straight line. As in Pourbaix diagrams, the separation limit between the acid and basic forms in water is a vertical line given by . The line separating the neutral acid in water and the anion Ao– in the organic phase is given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pourbaix Diagram
In electrochemistry, and more generally in solution chemistry, a Pourbaix diagram, also known as a potential/pH diagram, EH–pH diagram or a pE/pH diagram, is a plot of possible thermodynamically stable phases (''i.e.'', at chemical equilibrium) of an aqueous electrochemical system. Boundaries (50 %/50 %) between the predominant chemical species (aqueous ions in solution, or solid phases) are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects. Beside potential and pH, the equilibrium concentrations are also dependent upon, e.g., temperature, pressure, and concentration. Pourbaix diagrams are commonly given at room temperature, atmospheric pressure, and molar concentrations of 10−6 and changing any of these parameters will yield a different diagram. The diagrams are named after Marcel Pourbaix (1904–1998), the Russian-bor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvani Potential
In electrochemistry, the Galvani potential (also called Galvani potential difference, or inner potential difference, Δφ, delta phi) is the electric potential difference between two points in the bulk of two phases. These phases can be two different solids (e.g., two metals joined together), or a solid and a liquid (e.g., a metal electrode submerged in an electrolyte). The Galvani potential is named after Luigi Galvani. Galvani potential between two metals First, consider the Galvani potential between two metals. When two metals are electrically isolated from each other, an arbitrary voltage difference may exist between them. However, when two different metals are brought into electronic contact, electrons will flow from the metal with a lower voltage to the metal with the higher voltage until the Fermi level of the electrons in the bulk of both phases are equal. The actual numbers of electrons that passes between the two phases is small (it depends on the capacitance betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it. Hygroscopics ''are'' attracted to water, but are not dissolved by water. Molecules A hydrophilic molecule or portion of a molecule is one whose interactions with water and other polar substances are more thermodynamically favorable than their interactions with oil or other hydrophobic solvents. They are typically charge-polarized and capable of hydrogen bonding. This makes these molecules soluble not only in water but also in other polar solvents. Hydrophilic molecules (and portions of molecules) can be contrasted with hydrophobic molecules (and portions of molecules). In some cases, both hydrophilic and hydrophobic properties occur in a single molecule. An example of these amphiph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Fig 1
Ionic or Ionian may refer to: Arts and entertainment * Ionic meter, a poetic metre in ancient Greek and Latin poetry * Ionian mode, a musical mode or a diatonic scale Places and peoples * Ionian, of or from Ionia, an ancient region in western Anatolia * Ionians, one of four major tribes of the ancient Greeks * Ionian Sea, part of the Mediterranean Sea * Ionian Islands, a group of islands in Greece Language * Ionic Greek, an ancient dialect of the Greek language Science and technology * Ionian, of or relating to Io, a moon of the planet Jupiter * Ionian stage, a proposed name for the now-defined Chibanian stage in stratigraphy. * Ionic, of or relating to an ion, an atom or molecule with a net electric charge * Ionic (mobile app framework), a software development kit * Ionic bonding, a type chemical bonding * Ionic compound, a chemical compound involving ionic bonding Other uses * Ionian Technologies, an American biotechnology company * Hull Ionians, an English rugby club ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nernst Equation
In electrochemistry, the Nernst equation is a Thermodynamics#Chemical thermodynamics, chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (half-cell or electrochemical cell, full cell reaction) from the standard electrode potential, Thermodynamic temperature, absolute temperature, the number of electrons involved in the redox, redox reaction, and Thermodynamic activity, activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation respectively. It was named after Walther Nernst, a German physical chemist who formulated the equation. Expression General form with chemical activities When an oxidizer () accepts a number ''z'' of electrons () to be converted in its reduced form (), the half-reaction is expressed as: : + ''z'' → The reaction quotient ('), also often called the ion activity product (''IAP''), is the ratio between the chemical activity, chemical activities (' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
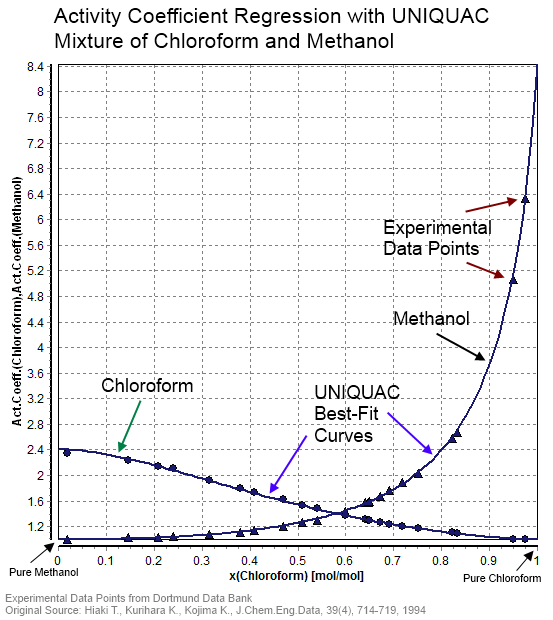
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\mu_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Fig 2
Ionic or Ionian may refer to: Arts and entertainment * Ionic meter, a poetic metre in ancient Greek and Latin poetry * Ionian mode, a musical mode or a diatonic scale Places and peoples * Ionian, of or from Ionia, an ancient region in western Anatolia * Ionians, one of four major tribes of the ancient Greeks * Ionian Sea, part of the Mediterranean Sea * Ionian Islands, a group of islands in Greece Language * Ionic Greek, an ancient dialect of the Greek language Science and technology * Ionian, of or relating to Io, a moon of the planet Jupiter * Ionian stage, a proposed name for the now-defined Chibanian stage in stratigraphy. * Ionic, of or relating to an ion, an atom or molecule with a net electric charge * Ionic (mobile app framework), a software development kit * Ionic bonding, a type chemical bonding * Ionic compound, a chemical compound involving ionic bonding Other uses * Ionian Technologies, an American biotechnology company * Hull Ionians, an English rugby club ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITIES
In electrochemistry, ITIES (interface between two immiscible electrolyte solutions) is an electrochemical interface that is either polarisable or polarised. An ITIES is polarisable if one can change the Galvani potential difference, or in other words the difference of inner potentials between the two adjacent phases, without noticeably changing the chemical composition of the respective phases (i.e. without noticeable electrochemical reactions taking place at the interface). An ITIES system is polarised if the distribution of the different charges and redox species between the two phases determines the Galvani potential difference. Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium tetraphenylborate dissolved in an organic solvent immiscible with water such as nitrobenzene, or 1,2-dichloroethane. Charge transfer reactions of an ITIES Three major ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |