|
Iodine 131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Camera
A gamma camera (γ-camera), also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy. The applications of scintigraphy include early drug development and nuclear medical imaging to view and analyse images of the human body or the distribution of medically injected, inhaled, or ingested radionuclides emitting gamma rays. Imaging techniques Scintigraphy ("scint") is the use of gamma cameras to capture emitted radiation from internal radioisotopes to create two-dimensional images. SPECT (single photon emission computed tomography) imaging, as used in nuclear cardiac stress testing, is performed using gamma cameras. Usually one, two or three detectors or heads, are slowly rotated around the patient's torso. Multi-headed gamma cameras can also be used for positron emission tomography (PET) scanning, provided that their hardware and software can be configured to detect "coincidences" (nea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Natural decay chain :\begin \ce \begin \ce \\ \ce \end \ce \\ \ce \begin \ce \\ \ce \end \ce \end Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/ mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature according ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
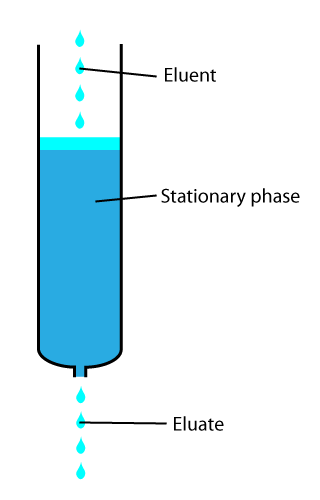
Eluted
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions. In a liquid chromatography experiment, for example, an analyte is generally adsorbed, or "bound to", an adsorbent in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Tellurium
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below. Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been found to be radioactive: 128Te and 130Te undergo double beta decay with half-lives of, respectively, 2.2×1024 (2.2 septillion) years (the longest half-life of all nuclides proven to be radioactive)Many isotopes are expected to have longer half-lives, but decay has not yet been observed in these, allowing only a lower limit to be placed on their half-lives and 8.2×1020 (820 quintillion) years. The longest-lived artificial radioisotope of tellurium is 121Te with a half-life of about 19 days. Several nuclear isomers have longer half-lives, the longest being 121mTe with a half-life of 154 days. The very-long-lived radioisotopes 128Te and 130Te are the two most common isotopes of tellurium. Of elements with at least one stable isotope, only i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to normal levels of background radiation. The term irradiation usually excludes the exposure to non-ionizing radiation, such as infrared, visible light, microwaves from cellular phones or electromagnetic waves emitted by radio and TV receivers and power supplies. Applications Sterilization If administered at appropriate levels, all forms of ionizing radiation can sterilize objects, including medical instruments, disposables such as syringes, and sterilize food. Ionizing radiation (electron beams, X-rays and gamma rays) may be used to kill bacteria in food or other organic material, including blood. Food irradiation, while effective, is seldom used due to problems with public accepta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation-induced Cancer
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation (which lies on the boundary between ionizing and non-ionizing radiation). Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydraulic Fracturing
Fracking (also known as hydraulic fracturing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of bedrock formations by a pressurized liquid. The process involves the high-pressure injection of "fracking fluid" (primarily water, containing sand or other proppants suspended with the aid of thickening agents) into a wellbore to create cracks in the deep-rock formations through which natural gas, petroleum, and brine will flow more freely. When the hydraulic pressure is removed from the well, small grains of hydraulic fracturing proppants (either sand or aluminium oxide) hold the fractures open. Hydraulic fracturing began as an experiment in 1947, and the first commercially successful application followed in 1950. As of 2012, 2.5 million "frac jobs" had been performed worldwide on oil and gas wells, over one million of those within the U.S. Such treatment is generally necessary to achieve adequate flow rates in shale gas, tight gas, tig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Tracer
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, use of radioisotope tracers are sometimes called radioisotope feeding experiments. Radioisotopes of hydrogen, carbon, phosphorus, sulfur, and iodine have been used extensively to trace the path of biochemical reactions. A radioactive tracer can also be used to track the distribution of a substance within a natural system such as a cell or tissue, or as a flow tracer to track fluid flow. Radioactive tracers are also used to determine the location of fractures created by hydraulic fracturing in natural gas production.Reis, John C. (1976). ''Environmental Cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IAEA
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 1957 as an autonomous organization within the United Nations system; though governed by its own founding treaty, the organization reports to both the General Assembly and the Security Council of the United Nations, and is headquartered at the UN Office at Vienna, Austria. The IAEA was created in response to growing international concern toward nuclear weapons, especially amid rising tensions between the foremost nuclear powers, the United States and the Soviet Union. U.S. President Dwight D. Eisenhower's " Atoms for Peace" speech, which called for the creation of an international organization to monitor the global proliferation of nuclear resources and technology, is credited with catalyzing the formation of the IAEA, whose treaty came into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xenon-135 is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)