|
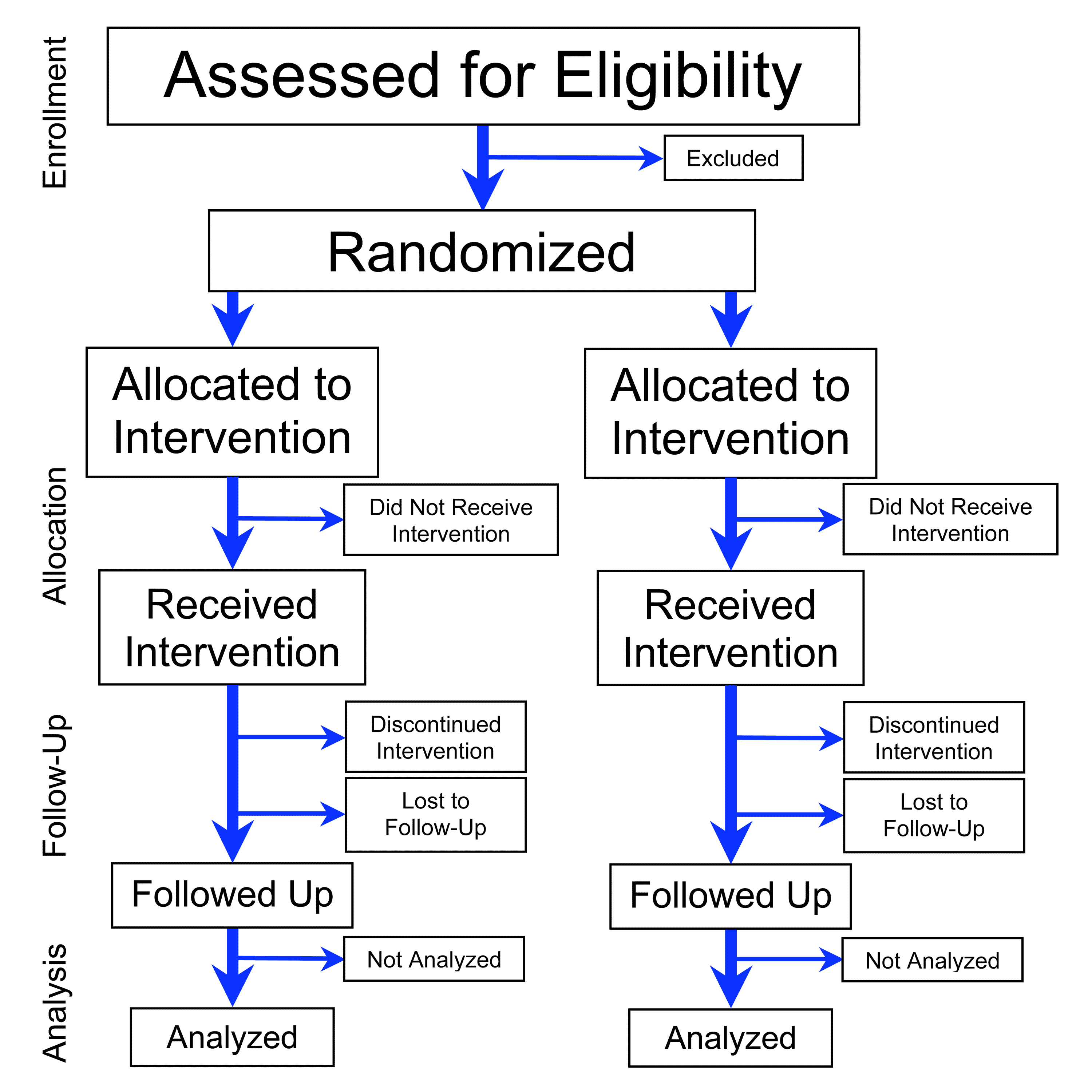
Intention To Treat
In medicine an intention-to-treat (ITT) analysis of the results of a randomized controlled trial is based on the initial treatment assignment and not on the treatment eventually received. ITT analysis is intended to avoid various misleading artifacts that can arise in intervention research such as non-random attrition of participants from the study or crossover. ITT is also simpler than other forms of study design and analysis, because it does not require observation of compliance status for units assigned to different treatments or incorporation of compliance into the analysis. Although ITT analysis is widely employed in published clinical trials, it can be incorrectly described and there are some issues with its application. Furthermore, there is no consensus on how to carry out an ITT analysis in the presence of missing outcome data. Rationale Randomized clinical trials analyzed by the intention-to-treat (ITT) approach provide unbiased comparisons among the treatment groups. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By Random assignment, randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing Standard of care#Medical standard of care, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I And Type II Errors
In statistical hypothesis testing, a type I error is the mistaken rejection of an actually true null hypothesis (also known as a "false positive" finding or conclusion; example: "an innocent person is convicted"), while a type II error is the failure to reject a null hypothesis that is actually false (also known as a "false negative" finding or conclusion; example: "a guilty person is not convicted"). Much of statistical theory revolves around the minimization of one or both of these errors, though the complete elimination of either is a statistical impossibility if the outcome is not determined by a known, observable causal process. By selecting a low threshold (cut-off) value and modifying the alpha (α) level, the quality of the hypothesis test can be increased. The knowledge of type I errors and type II errors is widely used in medical science, biometrics and computer science. Intuitively, type I errors can be thought of as errors of ''commission'', i.e. the researcher unluck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidemiology
Epidemiology is the study and analysis of the distribution (who, when, and where), patterns and determinants of health and disease conditions in a defined population. It is a cornerstone of public health, and shapes policy decisions and evidence-based practice by identifying risk factors for disease and targets for preventive healthcare. Epidemiologists help with study design, collection, and statistical analysis of data, amend interpretation and dissemination of results (including peer review and occasional systematic review). Epidemiology has helped develop methodology used in clinical research, public health studies, and, to a lesser extent, basic research in the biological sciences. Major areas of epidemiological study include disease causation, transmission, outbreak investigation, disease surveillance, environmental epidemiology, forensic epidemiology, occupational epidemiology, screening, biomonitoring, and comparisons of treatment effects such as in clinical trials. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protocol (natural Sciences)
In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other laboratories. Additionally, and by extension, protocols have the advantage of facilitating the assessment of experimental results through peer review. In addition to detailed procedures, equipment, and instruments, protocols will also contain study objectives, reasoning for experimental design, reasoning for chosen sample sizes, safety precautions, and how results were calculated and reported, including statistical analysis and any rules for predefining and documenting excluded data to avoid bias. Similarly, a protocol may refer to the procedural methods of health organizations, commercial laboratories, manufacturing plants, etc. to ensure their activities (e.g., bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature Clinical Practice
This is a list of journals published by Nature Research. These include the flagship ''Nature'' journal, the ''Nature Reviews'' series (which absorbed the former ''Nature Clinical Practice'' series in 2009), the ''npj'' series, ''Scientific Reports'' and many others. List A B C E G H I J L M N ;''Nature Reviews'' series ;''npj'' series The ''Nature Partner Journals'' series, abbreviated ''npj'', is a series of online-only, open access, journals. It was launched in April 2014 with three journals: ''npj Primary Care Respiratory Medicine'', ''npj Biofilms and Microbiomes'', and ''npj Schizophrenia''. Each journal in the series is published through a partnership between Springer Nature and a separate academic organization, foundation, or institution. O P S T References {{reflist External linksList of journalson Nature.com * Nature Research Nature Portfolio (formerly known as Nature Publishing Group and Nature Research) is a division of the intern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analysis Of Clinical Trials
Clinical trials are medical research studies conducted on human subjects. The human subjects are assigned to one or more interventions, and the investigators evaluate the effects of those interventions. The progress and results of clinical trials are analyzed statistically. Analysis factors Intention to treat Randomized clinical trials analyzed by the intention-to-treat (ITT) approach provide fair comparisons among the treatment groups because it avoids the bias associated with the non-random loss of the participants. The basic ITT principle is that participants in the trials should be analysed in the groups to which they were randomized, regardless of whether they received or adhered to the allocated intervention. However, medical investigators often have difficulties in accepting ITT analysis because of clinical trial issues like missing data or adherence to protocol. Per protocol This analysis can be restricted to only the participants who fulfill the protocol in terms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lost To Follow-up
In the clinical research trial industry, loss to follow-up refers to patients who at one point in time were actively participating in a clinical research trial, but have become lost (either by error in a computer tracking system or by being unreachable) at the point of follow-up in the trial. These patients can become lost for many reasons. Without properly informing the investigator associated with the clinical trial, they may have opted to withdraw from the clinical trial, moved away from the particular study site during the clinical trial, become ill and unable to communicate, are missing or are deceased. Adverse effects Patients who become lost to follow-up during a clinical research trial result in many negative effects on the outcome of the trial and on the pharmaceutical company sponsoring the clinical research trial. Patients who are lost-to-follow-up lead to incomplete study results, which in turn can put a bias on the result of the study as well as a bias on the inve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attrition (medicine, Epidemiology)
In science, attrition are ratios regarding the loss of participants during an experiment. Attrition rates are values that indicate the participant drop out. Higher attrition rates are found in longitudinal studies. See also * Intention-to-treat analysis In medicine an intention-to-treat (ITT) analysis of the results of a randomized controlled trial is based on the initial treatment assignment and not on the treatment eventually received. ITT analysis is intended to avoid various misleading artifac ... * Selection bias#Attrition Further reading * * {{cite book , first=Jeffrey M. , last=Wooldridge , title=Econometric Analysis of Cross Section and Panel Data , location=Cambridge , publisher=MIT Press , year=2010 , pages=837–842 , url=https://books.google.com/books?id=hSs3AgAAQBAJ&pg=PA837 , isbn=9780262296793 Experiments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conflicts Of Interest
A conflict of interest (COI) is a situation in which a person or organization is involved in multiple interests, financial or otherwise, and serving one interest could involve working against another. Typically, this relates to situations in which the personal interest of an individual or organization might adversely affect a duty owed to make decisions for the benefit of a third party. An "interest" is a commitment, obligation, duty or goal associated with a particular social role or practice. By definition, a "conflict of interest" occurs if, within a particular decision-making context, an individual is subject to two coexisting interests that are in direct conflict with each other. Such a matter is of importance because under such circumstances the decision-making process can be disrupted or compromised in a manner that affects the integrity or the reliability of the outcomes. Typically, a conflict of interest arises when an individual finds themselves occupying two soc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industry Sponsorship
Industry may refer to: Economics * Industry (economics), a generally categorized branch of economic activity * Industry (manufacturing), a specific branch of economic activity, typically in factories with machinery * The wider industrial sector of an economy, including manufacturing and production of other intermediate or final goods * The general characteristics and production methods common to an industrial society ** Industrialization, the transformation into an industrial society * Industry classification, a classification of economic organizations and activities Places *Industry, Alabama *Industry, California ** Industry station *Industry, Illinois *Industry, Kansas *Industry, Maine * Industry, Missouri *Industry, New York *Industry, Pennsylvania *Industry, Texas *Industry Bar, a New York City gay bar *Industry-Rock Falls Township, Phelps County, Nebraska Film and television * ''Made in Canada'' (TV series), a Canadian situation comedy series also known as ''The Industry'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)

