|
Inherent Chirality
In chemistry, inherent chirality is a property of asymmetry in molecules arising, not from a stereogenic or chiral center, but from a twisting of the molecule in 3-D space. The term was first coined by Volker Boehmer in a 1994 review, to describe the chirality of calixarenes arising from their non-planar structure in 3-D space. This phenomenon was described as resulting from "the absence of a place of symmetry or an inversion center in the molecule as a whole". Boehmer further explains this phenomenon by suggesting that if an inherently chiral calixarene macrocycle were opened up it would produce an "achiral linear molecule". There are two commonly used notations to describe a molecules inherent chirality: cR/cS (arising from the notation used for classically chiral compounds, with ''c'' denoting curvature) and P/M. Inherently chiral molecules, like their classically chiral counterparts, can be used in chiral host–guest chemistry, enantioselective synthesis, and other applicati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calixarenes
A calixarene is a macrocycle or Cyclic compound, cyclic oligomer based on a Methylene group, methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry. Nomenclature Calixarene IUPAC nomenclature, nomenclature is straightforward and involves counting the number of repeating units in the ring and including it in the name. A calix[4]arene has 4 units in the ring and a calix[6]arene has 6. A substituent in the meso position Rb is added to the name with a prefix C- as in C-methylcalix[6]arene The word calixarene is derived from the Greek calix or Chalice (cup), chalice because this type of molecule resembles a vase (or cup) and from the word arene that refers to the aromatic building block. Synthesis Calixarenes are generally produced by condensation of two components: an electron-rich aromatic compound, classically a 4-substituted phenol, and an aldehyde, classically forma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction in rods. Rhodopsin mediates dim light vision and thus is extremely sensitive to light. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which the rods are more sensitive. Defects in the rhodopsin gene cause eye diseases such as retinitis pigmentosa and congenital stationary night blindness. Names Rhodopsin was discovered by Franz Christian Boll in 1876. The name rhodospsin derives from Ancient Greek () for "rose", due to its pinkish color, and () for "sight". It was coined in 1878 by the German physiologist Wilhelm Friedrich Kühne (1837-1900). When George Wald discovered that rhodopsin is a holoprotein, consisting of retinal and an apoprotein, he c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry. Nomenclature Calixarene nomenclature is straightforward and involves counting the number of repeating units in the ring and including it in the name. A calix rene has 4 units in the ring and a calix rene has 6. A substituent in the meso position Rb is added to the name with a prefix C- as in C-methylcalix rene The word calixarene is derived from the Greek calix or chalice because this type of molecule resembles a vase (or cup) and from the word arene that refers to the aromatic building block. Synthesis Calixarenes are generally produced by condensation of two components: an electron-rich aromatic compound, classically a 4-substituted phenol, and an aldehyde, classically formaldehyde. *The scope for the aromatic component is broad diverse. The key at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
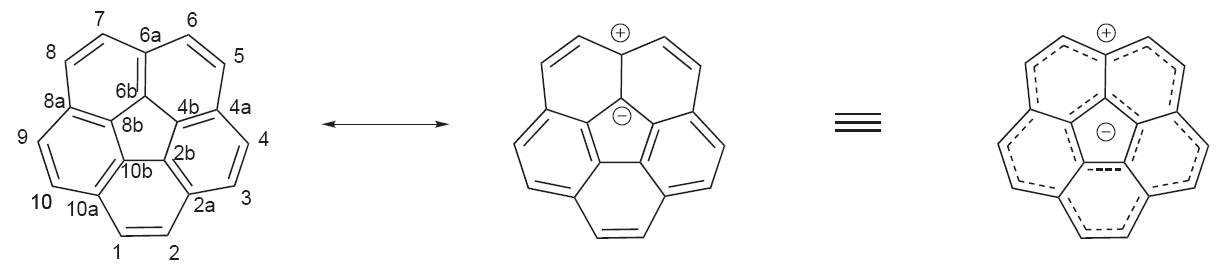
2corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/ mol (42.7 kJ/mol) at −64 °C. Synthesis Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. In 1971, the synthesis and properties of corannulane were reported. A flash vacuum pyrolysis method followed in 1991. One synthesis based on solution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/ mol (42.7 kJ/mol) at −64 °C. Synthesis Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulane was first isolated in 1966 by multistep organic synthesis. In 1971, the synthesis and properties of corannulane were reported. A flash vacuum pyrolysis method followed in 1991. One synthesis based on solution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planar Chirality
Planar chirality, also known as 2D chirality, is the special case of chirality for two dimensions. Most fundamentally, planar chirality is a mathematical term, finding use in chemistry, physics and related physical sciences, for example, in astronomy, optics and metamaterials. Recent occurrences in latter two fields are dominated by microwave and terahertz applications as well as micro- and nanostructured planar interfaces for infrared and visible light. In chemistry This term is used in chemistry contexts, e.g., for a chiral molecule lacking an asymmetric carbon atom, but possessing two non-coplanar rings that are each dissymmetric and which cannot easily rotate about the chemical bond connecting them: 2,2'-dimethylbiphenyl is perhaps the simplest example of this case. Planar chirality is also exhibited by molecules like (''E'')-cyclooctene, some di- or poly-substituted metallocenes, and certain monosubstituted paracyclophanes. Nature rarely provides planar chiral molecules, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Axial Chirality
{{disambiguation ...
Axial may refer to: * one of the anatomical directions describing relationships in an animal body * In geometry: :* a geometric term of location :* an axis of rotation * In chemistry, referring to an axial bond * a type of modal frame, in music * axial-flow, a type of fan * the Axial age in China, India, etc. * Axial Seamount and submarine volcano off Oregon, USA * Axial, Colorado, a ghost town See also *Axiality (other) *Axis (other) An axis (plural ''axes'') is an imaginary line around which an object rotates or is symmetrical. Axis may also refer to: Mathematics * Axis of rotation: see rotation around a fixed axis *Axis (mathematics), a designator for a Cartesian-coordinate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (havi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |