|
Infrared Chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊, : + -> lozenge -> roducts+ light For example, if is luminol and is hydrogen peroxide in the presence of a suitable catalyst we have: :\underset + \underset -> 3-APAlozenge-> + light where: * 3-APA is 3-aminophthalate * 3-APA ''◊is the vibronic excited state fluorescing as it decays to a lower energy level. General description The decay of this excited state ''◊to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to the Avogadro number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency. In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemoluminescent Reaction
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊, : + -> lozenge -> roducts+ light For example, if is luminol and is hydrogen peroxide in the presence of a suitable catalyst we have: :\underset + \underset -> 3-APAlozenge-> + light where: * 3-APA is 3-aminophthalate * 3-APA ''◊is the vibronic excited state fluorescing as it decays to a lower energy level. General description The decay of this excited state ''◊to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to the Avogadro number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency. In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). The term ''exothermic'' was first coined by 19th-century French chemist Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy usually in the form of heat. The concept is frequently applied in the physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Two types of chemical reactions Exothermic and endothermic describe two types of chemical reactions or systems found in nature, as follows: Exothermic After an exothermic reaction, more energy has been released to the surroundings than was absorbed to initiate and maintain the reaction. An example would be the burn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
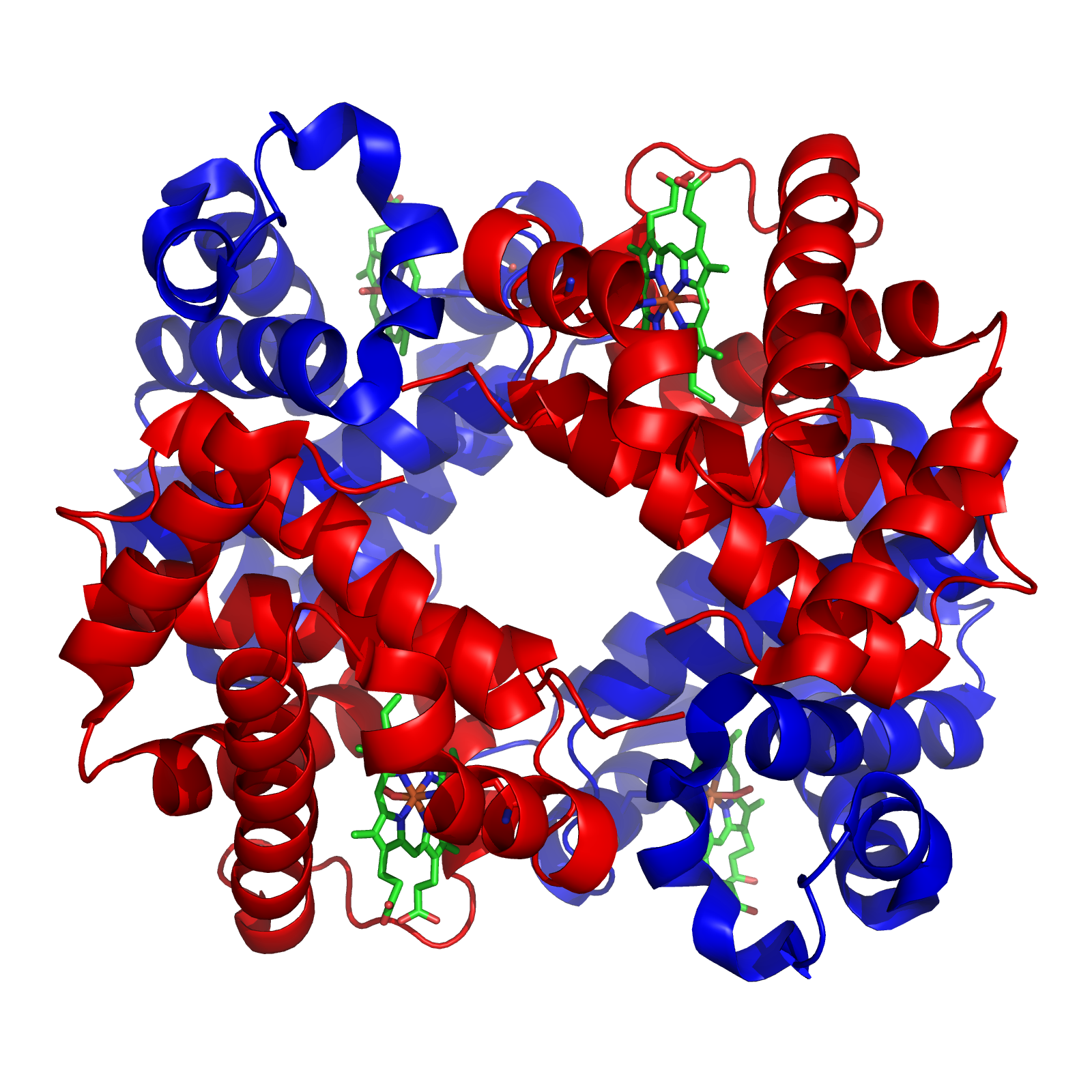
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in vertebrate blo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lampyris Noctiluca (firefly) Mating
''Lampyris'' is a genus of beetles in the Lampyridae. In most of western Eurasia, they are the predominant members of this family and includes the European common glow-worm, which is the type species. They produce a continuous glow;Stanger-Hall, Kathrin F.; Lloyd, James E. & Hillis, David M. (2007): Phylogeny of North American fireflies (Coleoptera: Lampyridae): Implications for the evolution of light signals. '' Mol. Phylogenet. Evol.'' 45(1): 33-49. PDF fulltext the larvae and larviform females are among those organisms commonly called "glowworms". This genus is rather close to '' Pleotomus'' and its relatives. These were formerly separated as tribe Pleotomini, but appear to be a specialized offshoot of the Lampyrini. Species ''BioLib'' includes the following species: * '' Lampyris algerica'' Ancey, 1869 * '' Lampyris ambigena'' Jacquelin du Val, 1860 * '' Lampyris angustula'' Fairmaire, 1895 * '' Lampyris brutia'' Costa, 1882 * '' Lampyris fuscata'' Geisthardt, 1987 * '' Lamp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemiluminescence
Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes. ECL is usually observed during application of potential (several volts) to electrodes of electrochemical cell that contains solution of luminescent species (polycyclic aromatic hydrocarbons, metal complexes, Quantum Dots or Nanoparticles ) in aprotic organic solvent (ECL compos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–750 nm) or infrared radiation (750–2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics. Concept Grotthuss–Draper law and Stark-Einstein law Photoexcitation is the first step in a photochemical process where the reactant is elevated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (electromagnetic Radiation)
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect is attenuation, or the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning synonymous with "absorption coefficient") * The Molar attenuation coefficient (a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is a conserved quantity carried by elementary particles, and thus by composite particles (hadrons) and atomic nucleus, atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being ''orbital angular momentum''. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical angular momentum of orbital revolution and appears when there is periodic structure to its wavefunction as the angle varies. For photons, spin is the quantum-mechanical counterpart of the Polarization (waves), polarization of light; for electrons, the spin has no classical counterpart. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The existence of the electron spin can also be inferred theoretically from the spin–statistics theorem and from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_mating.gif)