|
Indiana Caverns
Indiana Caverns is part of the Binkley Cave system near Corydon, Indiana. History In 1918, an 81.5 acre farm less than a mile south of Corydon was purchased by Arvel H. Miles. A sinkhole entrance to Binkley Cave opened on the farm at some point prior to a set of explorations in the 1930s. In 1944, the farm was purchased by Harvey Binkley, for whom the cave is now named. Most of the exploration of the cave has been done by the Indiana Speleological Survey. In 2010, Shane Myles and Tim Pride discovered an area now called Blowing Hole Boulevard. Blowing Hole Boulevard's west end is an eighty-five-foot tall room called Big Bone Mountain, named for several sets of animal bones found in the room. Subsequent excavations by the Indiana State Museum have determined the bones to originate from Pleistocene Ice Age animals. In 2012, the Indiana Speleological Survey began investigating a possible connection between Binkley Cave and nearby Blowing Hole Cave. A passageway thought to connect th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indiana
Indiana () is a U.S. state in the Midwestern United States. It is the 38th-largest by area and the 17th-most populous of the 50 States. Its capital and largest city is Indianapolis. Indiana was admitted to the United States as the 19th state on December 11, 1816. It is bordered by Lake Michigan to the northwest, Michigan to the north, Ohio to the east, the Ohio River and Kentucky to the south and southeast, and the Wabash River and Illinois to the west. Various indigenous peoples inhabited what would become Indiana for thousands of years, some of whom the U.S. government expelled between 1800 and 1836. Indiana received its name because the state was largely possessed by native tribes even after it was granted statehood. Since then, settlement patterns in Indiana have reflected regional cultural segmentation present in the Eastern United States; the state's northernmost tier was settled primarily by people from New England and New York, Central Indiana by migrants fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicious
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of arago ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolomite (mineral)
Dolomite () is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite. An alternative name sometimes used for the dolomitic rock type is dolostone. History As stated by Nicolas-Théodore de Saussure the mineral dolomite was probably first described by Carl Linnaeus in 1768. In 1791, it was described as a rock by the French naturalist and geologist Déodat Gratet de Dolomieu (1750–1801), first in buildings of the old city of Rome, and later as samples collected in the mountains now known as the Dolomite Alps of northern Italy. Nicolas-Théodore de Saussure first named the mineral (after Dolomieu) in March 1792. Properties The mineral dolomite crystallizes in the trigonal-rhombohedral system. It forms white, tan, gray, or pink crystals. Dolomite is a double carbonate, having an alternating structural arrangement of calcium and magnesium ions. Unless it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word ''gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum (calcined gypsum) used for various purposes, this dehydrated gypsum became known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric Oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide. Structure Fe2O3 can be obtained in various polymorphs. In the main one, α, iron adopts octahedral coordination geometry. That is, each Fe center is bound to six oxygen ligands. In the γ polymorph, some of the Fe sit on tetrahedral sites, with four oxygen ligands. Alpha phase α-Fe2O3 has the rhombohedral, corundum (α-Al2O3) structure and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. is α polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (this polymorph is called pyrolusite or ), with three-coordinate oxide and octahedral metal centres. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Sulfate
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
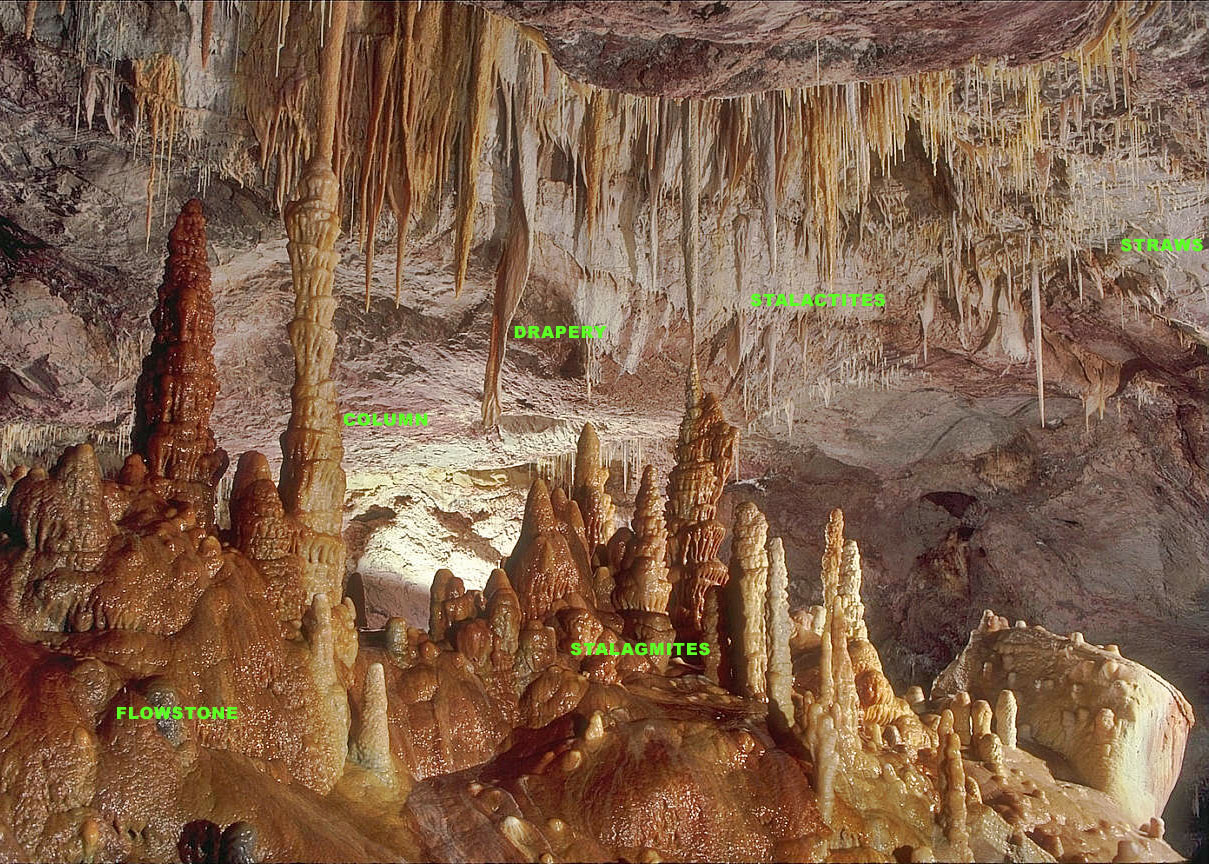
Flowstone
Flowstones are sheetlike deposits of calcite or other carbonate minerals, formed where water flows down the walls or along the floors of a cave. They are typically found in "solution caves", in limestone, where they are the most common speleothem. However, they may form in any type of cave where water enters that has picked up dissolved minerals. Flowstones are formed via the degassing of vadose percolation waters. Flowstone may also form on manmade structures as a result of calcium hydroxide being leached from concrete, lime or mortar. These secondary deposits created outside the cave environment, which mimic the shapes and forms of speleothems, are classified as "calthemites" and are associated with concrete degradation.Smith, G.K., (2016). “Calcite Straw Stalactites Growing From Concrete Structures”, Cave and Karst Science, Vol.43, No.1, P.4-10, (April 2016), British Cave Research Association, ISSN 1356-191X. Formation Flowing films of water that move along floors or do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helictites
A helictite is a speleothem (cave-formed mineral) found in a limestone cave that changes its axis from the vertical at one or more stages during its growth. Helictites have a curving or angular form that looks as if they were grown in zero gravity. They are most likely the result of capillary forces acting on tiny water droplets, a force often strong enough at this scale to defy gravity. Helictites are, perhaps, the most delicate of cave formations. They are usually made of needle-form calcite and aragonite. Helictite forms have been described in several types: ribbon helictites, saws, rods, butterflies, "hands", curly-fries, and "clumps of worms". They typically have radial symmetry. They can be easily crushed or broken by the slightest touch. Because of this, helictites are rarely seen within arm's reach in tourist caves. Timpanogos Cave National Monument in Utah has one of the largest collections of these formations in the world. Large numbers are also in the Jenolan Cave ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalagmites
A stalagmite (, ; from the Greek , from , "dropping, trickling") is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Mnemonics have been developed for which word refers to which type of formation; one is that ''stalactite'' has a C for "ceiling", and ''stalagmite'' has a G for "ground", another is that, as with ants in the pants, the mites go up and the tights (tites) come down. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carbonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |