|
Iclaprim
Iclaprim is an antibiotic drug candidate that is active against Gram positive organisms. It is administered intravenously. ''In vitro'', iclaprim is active against methicillin-resistant ''Staphylococcus aureus'' (MRSA), vancomycin-resistant ''Staphylococcus aureus'' (VRSA), strains of ''Streptococcus pneumoniae'' resistant to several common antibiotics, and some Gram-negative bacteria. It is of the diaminopyrimidine dihydrofolate reductase (DHFR)- inhibiting type. History Iclaprim is an optimized analog of trimethoprim that was discovered by scientists at Roche. Arpida was spun out of Roche in 1998, and acquired iclaprim from Roche in 2001. Arpida held an initial public offering on the Swiss stock exchange in 2005. Arpida ran two Phase III clinical trials for complicated skin and skin structure infections that were completed by 2008, but as of 2017, had not been published in the medical literature. A new drug application was filed with the United States Food and Drug Admin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diaminopyrimidine
Diaminopyrimidines (DAP) are a class of organic chemical compounds that include two amine groups on a pyrimidine ring. They include many dihydrofolate reductase inhibitor drugs (such as pyrimethamine, trimetrexate, and piritrexim and the antibiotics Iclaprim and trimethoprim). Some have been patented as anti-cancer drugs. See also * 2,4-Diaminopyrimidine * 4,5-Diaminopyrimidine 4,5-Diaminopyrimidine is a diaminopyrimidine. See also * 2,4-Diaminopyrimidine 2,4-Diaminopyrimidine is a diaminopyrimidine Diaminopyrimidines (DAP) are a class of organic chemical compounds that include two amine groups on a pyrimidine ring. ... References Aminopyrimidines {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethoprim
Trimethoprim (TMP) is an antibiotic used mainly in the treatment of bladder infections. Other uses include for middle ear infections and travelers' diarrhea. With sulfamethoxazole or dapsone it may be used for ''Pneumocystis'' pneumonia in people with HIV/AIDS. It is taken by mouth. Common side effects include nausea, changes in taste, and rash. Rarely it may result in blood problems such as not enough platelets or white blood cells. Trimethoprim may cause sun sensitivity. There is evidence of potential harm during pregnancy in some animals but not humans. It works by blocking folate metabolism via dihydrofolate reductase in some bacteria which results in their death. Trimethoprim was first used in 1962. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. Medical uses It is primarily used in the treatment of urinary tract infections, although it may be used against any susceptible aerobic bacterial species. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic Mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug-induced QT Prolongation
QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. It is an electrical disturbance which can be seen on an electrocardiogram (ECG). Excessive QT prolongation can trigger tachycardias such as torsades de pointes (TdP). QT prolongation is an established side effect of antiarrhythmics, but can also be caused by a wide range of non-cardiac medicines, including antibiotics, antihistamines, opioids, and complementary medicines. On an ECG, the QT interval represents the summation of action potentials in cardiac muscle cells, which can be caused by an increase in inward current through sodium or calcium channels, or a decrease in outward current through potassium channels. By binding to and inhibiting the “rapid” delayed rectifier potassium current protein, certain drugs are able to decrease the outward flow of potassium ions and extend the length of phase 3 myocardial repolarization, res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzopyrans
Benzopyran is a polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring. According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2''H''-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues. There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature. Some benzopyrans have shown anticancerous activity ''in vitro''. The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterial Dihydrofolate Reductase Inhibitors
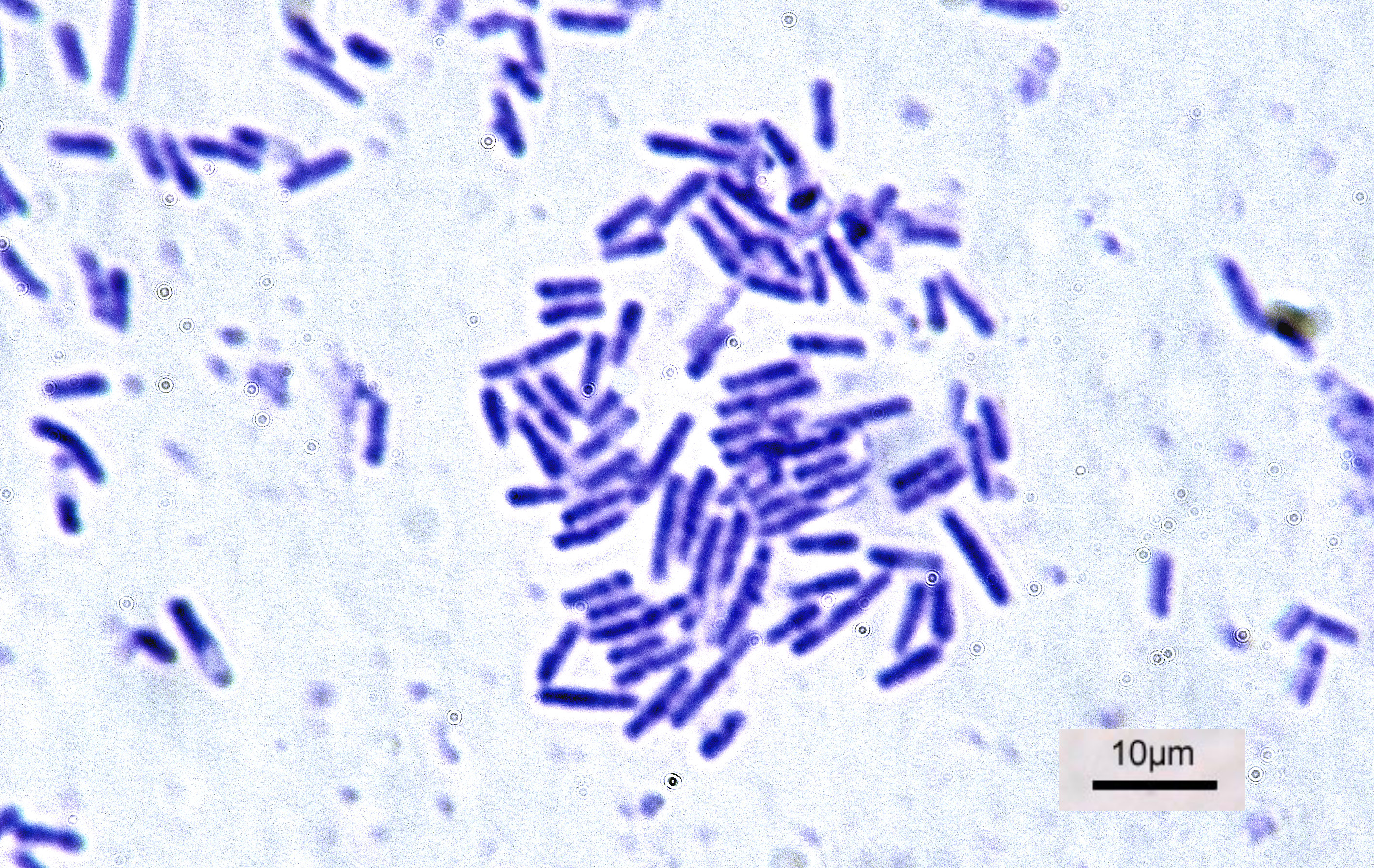
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationships wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemate
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant ''Staphylococcus aureus''. Blood levels may be measured to determine the correct dose. Vancomycin is also taken by mouth as a treatment for severe ''Clostridium difficile'' colitis. When taken by mouth it is poorly absorbed. Common side effects include pain in the area of injection and allergic reactions. Occasionally, hearing loss, low blood pressure, or bone marrow suppression occur. Safety in pregnancy is not clear, but no evidence of harm has been found, and it is likely safe for use when breastfeeding. It is a type of glycopeptide antibiotic and works by blocking the construction of a cell wall. Vancomycin was approved for medical use in the United States in 1958. It is on the Wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |