|
Ice Sluice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. In the Solar System, ice is abundant and occurs naturally from as close to the Sun as Mercury to as far away as the Oort cloud objects. Beyond the Solar System, it occurs as interstellar ice. It is abundant on Earth's surfaceparticularly in the polar regions and above the snow lineand, as a common form of precipitation and deposition, plays a key role in Earth's water cycle and climate. It falls as snowflakes and hail or occurs as frost, icicles or ice spikes and aggregates from snow as glaciers and ice sheets. Ice exhibits at least eighteen phases ( packing geometries), depending on temperature and pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form depending on its hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canal Park, Duluth
Canal Park is a tourist and recreation-oriented district of Duluth, Minnesota, Duluth, Minnesota, United States. Situated across the Interstate 35 in Minnesota, Interstate 35 freeway from Downtown Duluth, it is connected by the Aerial Lift Bridge across the Duluth Ship Canal to the Minnesota Point, Park Point sandbar and Neighborhoods of Duluth, Minnesota, neighborhood. Canal Park Drive and Lake Avenue South serve as the main routes in Canal Park. History Canal Park is largely a conversion of an old warehouse district into restaurants, shops (especially those dealing in antiques and other novelties), cafés, and hotels. This conversion began in the 1980s as an attempt to use Duluth's rich Industrial sector, industrial past, the decline of which had left the city in economic turmoil at the time, as an asset in a prospective tourist industry. Attractions Many annual events are held in the Canal Park area, such as the Bayfront Blues Festival and Grandma's Marathon, which starts in Tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice, fresh water, saline water (salt water) and atmospheric water is variable depending on a wide range of climatic variables. The water moves from one reservoir to another, such as from river to ocean, or from the ocean to the atmosphere, by the physical processes of evaporation, transpiration, condensation, precipitation, infiltration, surface runoff, and subsurface flow. In doing so, the water goes through different forms: liquid, solid (ice) and vapor. The ocean plays a key role in the water cycle as it is the source of 86% of global evaporation. The water cycle involves the exchange of energy, which leads to temperature changes. When water evaporates, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
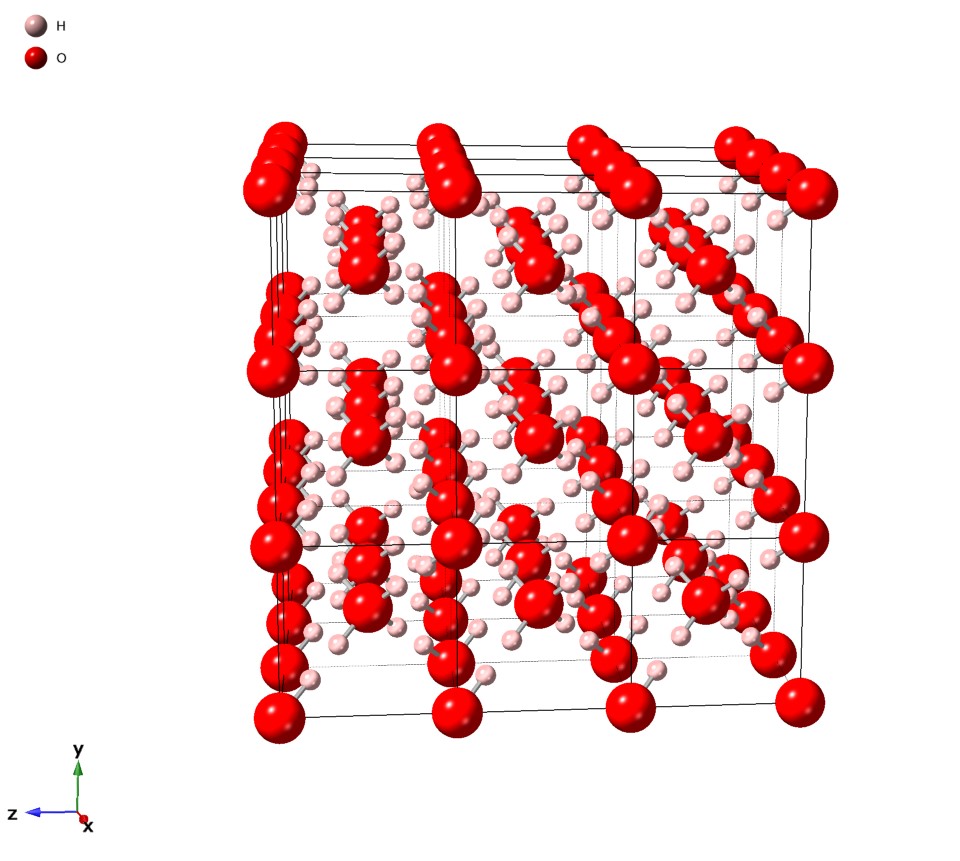
Ice VII
Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above . Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt. Like the majority of ice phases (including ice Ih), the hydrogen atom positions are dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Ic
Ice Ic (pronounced "ice one c" or "ice I see") is a metastable cubic crystalline variant of ice. Hans König was the first to identify and deduce the structure of ice Ic. The oxygen atoms in ice Ic are arranged in a diamond structure; it is extremely similar to ice Ih having nearly identical densities and the same lattice constant along the hexagonal puckered-planes. It forms at temperatures between upon cooling, and can exist up to upon warming, when it transforms into ice Ih. Apart from forming from supercooled water, ice Ic has also been reported to form from amorphous ice as well as from the high-pressure ices II, III and V. It can form in and is occasionally present in the upper atmosphere and is believed to be responsible for the observation of Scheiner's halo, a rare ring that occurs near 28 degrees from the Sun or the Moon. Ordinary water ice is known as ice Ih (in the Bridgman nomenclature). Different types of ice, from ice II to ice XIX, have been created in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
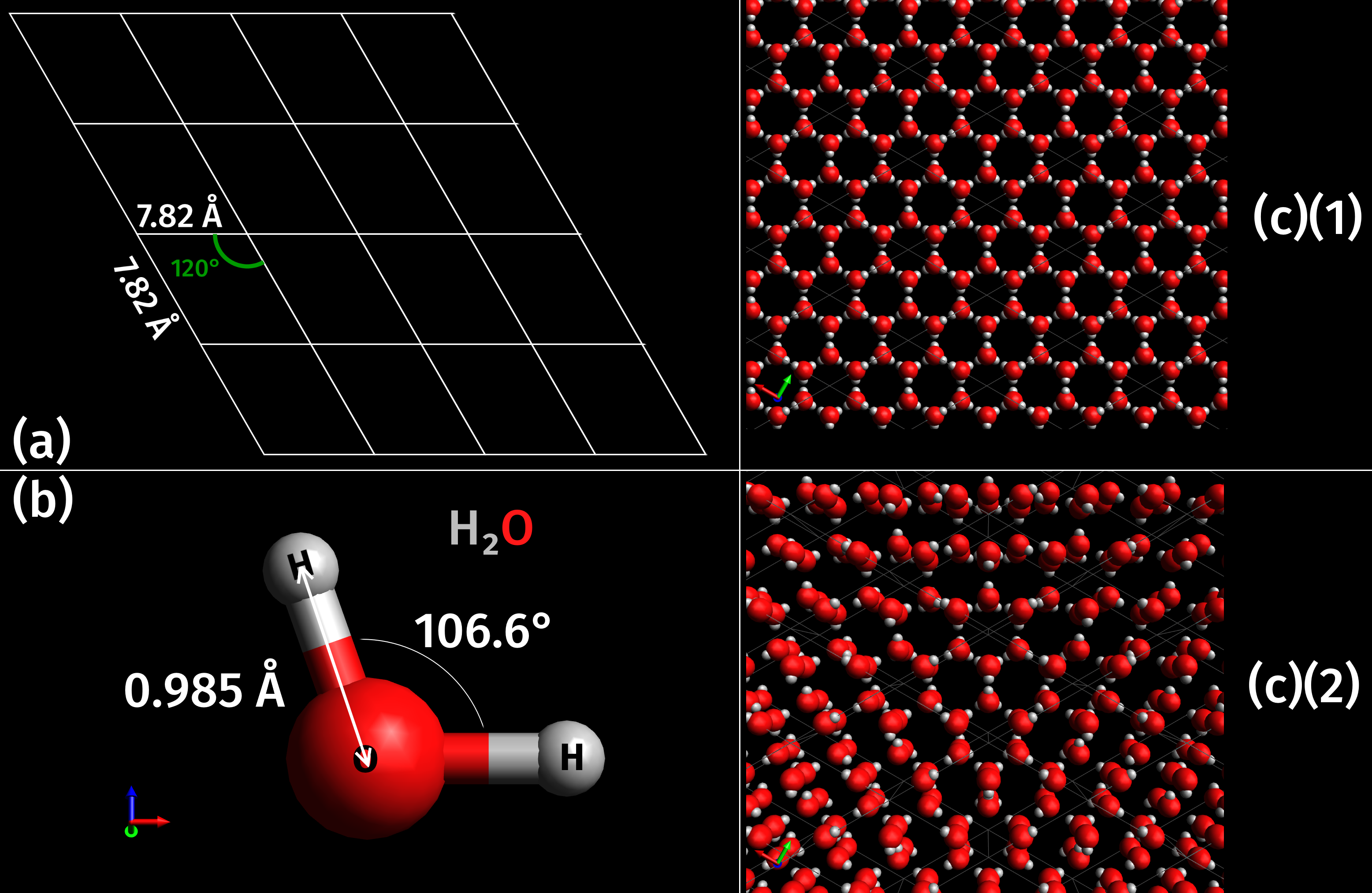
Ice Ih
Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on Earth. Phase space of ice Ih with respect to other ice phases. Ice Ih (hexagonal ice crystal) (pronounced: ice one h, also known as ice-phase-one) is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic that is occasionally present in the upper atmosphere. Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate. For a description of these properties, see ''Ice'', which deals primarily with ice Ih. The crystal structure is characterized by the oxygen atoms forming hexagonal symmetry with near tetrahedral bonding angles. Ice Ih is stable down to , as evidenced by x-ray diffraction and extremely high resolution thermal expansion measurements. Ice Ih is also stable under applied pressures of up to about where i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macroscopic Quantum Phenomena
Macroscopic quantum phenomena are processes showing quantum behavior at the macroscopic scale, rather than at the atomic scale where quantum effects are prevalent. The best-known examples of macroscopic quantum phenomena are superfluidity and superconductivity; other examples include the quantum Hall effect and topological order. Since 2000 there has been extensive experimental work on quantum gases, particularly Bose–Einstein condensates. Between 1996 and 2016 six Nobel Prizes were given for work related to macroscopic quantum phenomena. Macroscopic quantum phenomena can be observed in superfluid helium and in superconductors, but also in dilute quantum gases, dressed photons such as polaritons and in laser light. Although these media are very different, they are all similar in that they show macroscopic quantum behavior, and in this respect they all can be referred to as quantum fluids. Quantum phenomena are generally classified as macroscopic when the quantum states are oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Ice
Amorphous ice (non-crystalline or "vitreous" ice) is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range order in its molecular arrangement. Amorphous ice is produced either by rapid cooling of liquid water (so the molecules do not have enough time to form a crystal lattice), or by compressing ordinary ice at low temperatures. Although almost all water ice on Earth is the familiar crystalline ice Ih, amorphous ice dominates in the depths of interstellar medium, making this likely the most common structure for H2O in the universe at large. Just as there are many different crystalline forms of ice (currently more than seventeen are known), there are also different forms of amorphous ice, distinguished principally by their densities. Formation The production of amorphous ice hinges on the fast rate of cooling. Liquid water must be cooled to its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable, and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness. In metallurgy, quenching is most commonly used to harden steel by inducing a martensite transformation, where the steel must be rapidly cooled through its eutectoid point, the temperature at which austenite becomes unstable. In steel alloyed with metals such as nickel and manganese, the eutectoid temperature becomes much lower, but the kinetic barriers to phase transformation remain the same. This allows quenching to start at a lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphere Packing
In geometry, a sphere packing is an arrangement of non-overlapping spheres within a containing space. The spheres considered are usually all of identical size, and the space is usually three-dimensional Euclidean space. However, sphere packing problems can be generalised to consider unequal spheres, spaces of other dimensions (where the problem becomes circle packing in two dimensions, or hypersphere packing in higher dimensions) or to non-Euclidean spaces such as hyperbolic space. A typical sphere packing problem is to find an arrangement in which the spheres fill as much of the space as possible. The proportion of space filled by the spheres is called the ''packing density'' of the arrangement. As the local density of a packing in an infinite space can vary depending on the volume over which it is measured, the problem is usually to maximise the average or asymptotic density, measured over a large enough volume. For equal spheres in three dimensions, the densest packing uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_(cropped).jpg)