|
INSIG1
Insulin induced gene 1, also known as INSIG1, is a protein which in humans is encoded by the ''INSIG1'' gene. INSIG1 is short for insulin-induced gene 1; it is located on chromosome 7 (7q36). This human gene encodes for a transmembrane protein of 277 amino acids with probably 6 transmembrane domains. It is localized in the endoplasmic reticulum (ER) and seems to be expressed in all tissues, especially in liver. This gene is called an insulin-induced gene because the molecule insulin can regulate it. Importantly, the protein encoded by this gene plays a critical role in regulating cholesterol concentrations in cells. Function #INSIG1 plays an important role in the SREBP-mediated regulation of cholesterol biosynthesis: by binding to the sterol-sensing domain of SCAP (SREBP cleavage activating protein) it makes the SCAP/SREBP complex stay longer in the ER, thus prohibiting SCAP from carrying activated SREBP to the golgi complex The Golgi apparatus (), also known as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
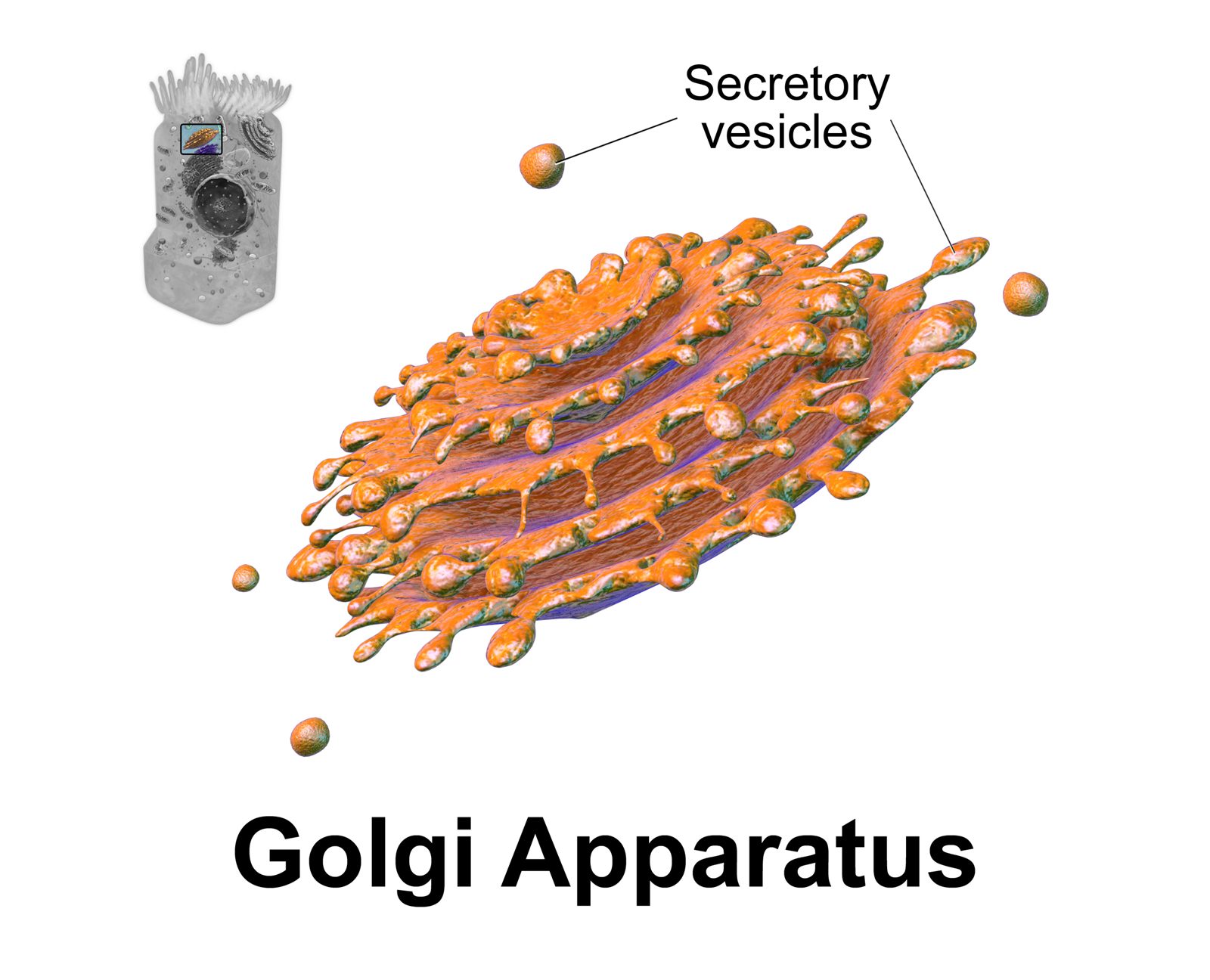
Golgi Complex
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG CoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol Regulatory Element Binding Protein
Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes ''SREBF1'' and ''SREBF2''. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors. Unactivated SREBPs are attached to the nuclear envelope and endoplasmic reticulum membranes. In cells with low levels of sterols, SREBPs are cleaved to a water-soluble N-terminal domain that is translocated to the nucleus. These activated SREBPs then bind to specific sterol regulatory element DNA sequences, thus upregulating the synthesis of enzymes involved in sterol biosynthesis. Sterols in turn inhibit the cleavage of SREBPs and therefore synthesis of additional sterols is reduced through a negative feed back loop. Isoforms Mammalian genomes have two separate SREBP genes ( and ): * SREBP-1 expression produces two different isoforms, SREBP-1a and -1c. These isoforms differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver X Receptor
The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands they were subsequently deorphanized. Two isoforms of LXR have been identified and are referred to as LXRα and LXRβ. The liver X receptors are classified into subfamily 1 (thyroid hormone receptor-like) of the nuclear receptor superfamily, and are given the nuclear receptor nomenclature symbols NR1H3 (LXRα) and NR1H2 (LXRβ) respectively. LXRα and LXRβ were discovered separately between 1994-1995. LXRα isoform was independently identified by two groups and initially named RLD-1 and LXR, whereas four groups identified the LXRβ isoform and called it UR, NER, OR-1, and RIP-15. The h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxysterol
An oxysterol is a derivative of cholesterol obtained by oxidation involving enzymes and / or pro-oxidants. Such compounds play important roles in various biological processes such as cholesterol homeostasis, lipid metabolism (sphingolipids, fatty acids), apoptosis, autophagy, and prenylation of proteins; the mode of action of oxysterols in these effects is still poorly understood. Several oxysterols are associated with age-related diseases such as cardiovascular disease, eye disease (cataract, age-related macular degeneration), certain neurodegenerative diseases and cancers. The activities of oxysterols in these diseases could be due to their pro-oxidative and pro-inflammatory activities and their ability to act on cellular organelles (mitochondria, peroxisome, lysosome) that can contribute to activate apoptosis and autophagy. There are arguments supporting that oxysterols have important roles in atherosclerosis progression which is involved in several cardiovascular diseases. Ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patched
Patched (Ptc) is a conserved 12-pass transmembrane protein receptor that plays an obligate negative regulatory role in the Hedgehog signaling pathway in insects and vertebrates. Patched is an essential gene in embryogenesis for proper segmentation in the fly embryo, mutations in which may be embryonic lethal. Patched functions as the receptor for the Hedgehog protein and controls its spatial distribution, in part via endocytosis of bound Hedgehog protein, which is then targeted for lysosomal degradation. Discovery The original mutations in the ''ptc'' gene were discovered in the fruit fly ''Drosophila melanogaster'' by 1995 Nobel Laureates Eric F. Wieschaus and Christiane Nusslein-Volhard and colleagues, and the gene was independently cloned in 1989 by Joan Hooper in the laboratory of Matthew P. Scott, and by Philip Ingham and colleagues. Role in hedgehog signaling Patched is part of a negative feedback mechanism for hedgehog signaling that helps shape the sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NPC1
Niemann-Pick disease, type C1 (NPC1) is a disease of a membrane protein that mediates intracellular cholesterol trafficking in mammals. In humans the protein is encoded by the ''NPC1'' gene (chromosome location 18q11). Function NPC1 was identified as the gene that when mutated, results in Niemann-Pick disease, type C. Niemann-Pick disease, type C is a rare neurovisceral lipid storage disorder resulting from autosomal recessively inherited loss-of-function mutations in either NPC1 or NPC2. This disrupts intracellular lipid transport, leading to the accumulation of lipid products in the late endosomes and lysosomes. Approximately 95% of NPC patients are found to have mutations in the NPC1 gene. NPC1 encodes a putative integral membrane protein containing sequence motifs consistent with a role in intracellular transport of cholesterol and sphingosine to post-lysosomal destinations. Clinical significance Obesity Mutations in the NPC1 gene have been strongly linked with ob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMGCoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SREBP Cleavage Activating Protein
Sterol regulatory element-binding protein cleavage-activating protein, also known as SREBP cleavage-activating protein or SCAP is a protein that in humans is encoded by the ''SCAP'' gene. SCAP contains a sterol-sensing domain (SSD) and seven WD domains. In cholesterol-depleted cells, this protein binds to sterol regulatory element binding proteins (SREBPs) and mediates their transport from the ER to the Golgi apparatus. The SREBPs are then proteolytically cleaved and stimulate sterol biosynthesis. Function SCAP is a regulatory protein that is required for the proteolytic cleavage of the sterol regulatory element-binding protein (SREBP). SCAP is an integral membrane protein located in the endoplasmic reticulum (ER). One of the cytosolic regions of SCAP contains a hexapeptide amino acid sequence, MELADL, that functions to detect cellular cholesterol. When cholesterol is present, SCAP undergoes a conformational change that prevents it from activating SREBP and cholesterol synth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol-sensing Domain
A sterol-sensing domain (SSD) is a protein domain which consists of 180 amino acids forming five transmembrane segments capable of binding sterol groups. This type of domain is present in proteins involved in cholesterol metabolism and signalling. Function Sterol-sensing domains are present in various proteins involved in key aspects of cholesterol homeostasis and signalling. Multiple sequence alignments using Clustal W have shown that these proteins can be grouped in seven different families according to their SSDs. The following SSD-containing proteins represent each family: *HMG-CoA reductase (HMGCR), involved in the biosynthesis of cholesterol. This was the first protein with an SSD to be discovered. Upon binding to cholesterol, this protein undergoes endoplasmic-reticulum-associated protein degradation. The SSD is not required for the catalytic activity of HMGCR. * SREBP cleavage-activating protein (SCAP), which regulates transcription of genes with sterol response elements b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |