|
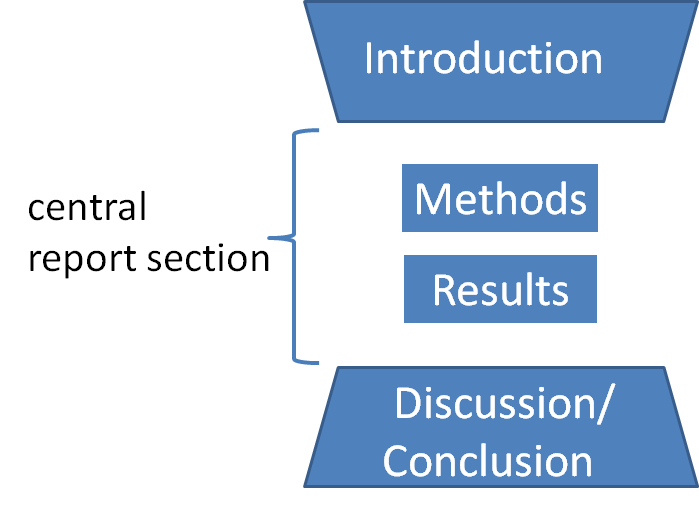
IMRAD
In scientific writing, IMRAD or IMRaD () (Introduction, Methods, Results, and Discussion) is a common organizational structure for the format of a document. IMRaD is the most prominent norm for the structure of a scientific journal article of the original research type. Overview Original research articles are typically structured in this basic order *Introduction – Why was the study undertaken? What was the research question, the tested hypothesis or the purpose of the research? *Methods – When, where, and how was the study done? What materials were used or who was included in the study groups (patients, etc.)? *Results – What answer was found to the research question; what did the study find? Was the tested hypothesis true? *Discussion – What might the answer imply and why does it matter? How does it fit in with what other researchers have found? What are the perspectives for future research? The plot and the flow of the story of the IMRaD style of writing are explained b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Abstract (summary)
An abstract is a brief summary of a research article, thesis, review, conference proceeding, or any in-depth analysis of a particular subject and is often used to help the reader quickly ascertain the paper's purpose. When used, an abstract always appears at the beginning of a manuscript or typescript, acting as the point-of-entry for any given academic paper or patent application. Abstracting and indexing services for various academic disciplines are aimed at compiling a body of literature for that particular subject. The terms ''précis'' or ''synopsis'' are used in some publications to refer to the same thing that other publications might call an "abstract". In management reports, an '' executive summary'' usually contains more information (and often more sensitive information) than the abstract does. Purpose and limitations Academic literature uses the abstract to succinctly communicate complex research. An abstract may act as a stand-alone entity instead of a full paper. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uniform Requirements For Manuscripts Submitted To Biomedical Journals
The ICMJE recommendations (full title, "Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals") are a set of guidelines produced by the International Committee of Medical Journal Editors for standardising the ethics, preparation and formatting of manuscripts submitted to biomedical journals for publication. Compliance with the ICMJE recommendations is required by most leading biomedical journals. Levels of real compliance are subject to debate. As of 9 January 2020, 5570 journals worldwide claim to follow the ICMJE recommendations.International Committee of Medical Journal Editors. Journals that have Requested Inclusion on the List of Publications that follow the ICMJE's Uniform Requirements For Manuscripts Submitted to Biomedical Journals [homepage on the Internet]. Philadelphia: ICMJE; c2005 [updated 27 May 2006; cited 30 May 2006]. Available from: http://www.icmje.org/journals.html The recommendations were first issued in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Scientific Journal
In academic publishing, a scientific journal is a periodical publication designed to further the progress of science by disseminating new research findings to the scientific community. These journals serve as a platform for researchers, scholars, and scientists to share their latest discoveries, insights, and methodologies across a multitude of scientific disciplines. Unlike professional or trade magazines, the articles are mostly written by scientists rather than staff writers employed by the journal. Scientific journals are characterized by their rigorous peer review process, which aims to ensure the validity, reliability, and quality of the published content. In peer review, submitted articles are reviewed by active scientists (peers) to ensure scientific rigor. With origins dating back to the 17th century, the publication of scientific journals has evolved significantly, advancing scientific knowledge, fostering academic discourse, and facilitating collaboration within ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Academic Journal
An academic journal (or scholarly journal or scientific journal) is a periodical publication in which Scholarly method, scholarship relating to a particular academic discipline is published. They serve as permanent and transparent forums for the dissemination, scrutiny, and discussion of research. Unlike professional magazines or Trade magazine, trade magazines, the articles are mostly written by researchers rather than staff writers employed by the journal. They nearly universally require peer review for Research Article, research articles or other scrutiny from contemporaries competent and established in their respective fields. Academic journals trace their origins back to the 17th century. , it is estimated that over 28,100 active academic journals are in publication, with scopes ranging from the general sciences, as seen in journals like ''Science (journal), Science'' and ''Nature (journal), Nature'', to highly specialized fields. These journals publish a variety of articles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Peter Medawar
Sir Peter Brian Medawar (; 28 February 1915 – 2 October 1987) was a British biologist and writer, whose works on graft rejection and the discovery of acquired immune tolerance have been fundamental to the medical practice of tissue and organ transplants. For his scientific works, he is regarded as the "father of transplantation". He is remembered for his wit both in person and in popular writings. Richard Dawkins referred to him as "the wittiest of all scientific writers"; Stephen Jay Gould as "the cleverest man I have ever known". Medawar was the youngest child of a Lebanese people, Lebanese father and a British mother, and was both a Brazilian and British citizen by birth. He studied at Marlborough College and Magdalen College, Oxford, and was professor of zoology at the University of Birmingham and University College London. Until he was partially disabled by a cerebral infarction, he was Director of the National Institute for Medical Research at Mill Hill. With his doctor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Institutional Animal Care And Use Committee
Institutional Animal Care and Use Committees (IACUCs) are centrally important in applying laws about animal research in the United States. Similar systems operate in other countries, but generally under different titles; for example, in Canada a typical title would be the University Animal Care Committee (UACC), while in the United Kingdom it would be the Animal Welfare and Ethical Review Body (AWERB). Most research involving laboratory animals in the United States is funded by the United States National Institutes of Health or, to lesser extents, other federal agencies. The NIH Office of Laboratory Animal Welfare (OLAW) has been directed by law to develop policies that describe the role of Institutional Animal Care and Use Committees. Every institution that uses certain animals for federally funded laboratory research must have an Institutional Animal Care and Use Committee (IACUC). Each local IACUC reviews research protocols and conducts evaluations of the institution's animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Institutional Review Board
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee at an institution that applies research ethics by reviewing the methods proposed for research involving human subjects, to ensure that the projects are ethical. The main goal of IRB reviews is to ensure that study participants are not harmed (or that harms are minimal and outweighed by research benefits). Such boards are formally designated to approve (or reject), monitor, and review biomedical and behavioral research involving humans, and they are legally required in some countries under certain specified circumstances. Most countries use some form of IRB to safeguard ethical conduct of research so that it complies with national and international norms, regulations or codes. The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of people participating in a research stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Declaration Of Helsinki
The Declaration of Helsinki (DoH, ) is a set of ethical principles regarding human experimentation developed originally in 1964 for the medical community by the World Medical Association (WMA). It is widely regarded as the cornerstone document on human research ethics. It is not a legally binding instrument under international law, but instead draws its authority from the degree to which it has been codified in, or influenced, national or regional legislation and regulations. Its role was described by a Brazilian forum in 2000 in these words: "Even though the Declaration of Helsinki is the responsibility of the World Medical Association, the document should be considered the property of all humanity." Principles The Declaration is morally binding on physicians, and that obligation overrides any national or local laws or regulations, if the Declaration provides for a higher standard of protection of humans than the latter. Investigators still have to abide by local legislation b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Conflicts Of Interest In Academic Publishing
Conflicts of interest (COIs) often arise in academic publishing. Such conflicts may cause wrongdoing and make it more likely. Ethical standards in academic publishing exist to avoid and deal with conflicts of interest, and the field continues to develop new standards. Standards vary between journals and are unevenly applied. According to the International Committee of Medical Journal Editors, "[a]uthors have a responsibility to evaluate the integrity, history, practices and reputation of the journals to which they submit manuscripts". Conflicts of interest increase the likelihood of biases arising; they can harm the quality of research and the public good (even if disclosed). Conflicts of interest can involve research sponsors, authors, journals, journal staff, publishers, and peer reviewers. Avoidance, disclosure, and tracking The avoidance of conflicts of interest and the changing of the structure of institutions to make them easier to avoid are frequently advocated for. Some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Subheading
News style, journalistic style, or news-writing style is the prose style used for news reporting in media, such as newspapers, radio, and television. News writing attempts to answer all the basic questions about any particular event—who, what, when, where, and why (the Five Ws) and often how—at the opening of the article. This form of structure is sometimes called the " inverted pyramid", to refer to the decreasing importance of information in subsequent paragraphs. News stories also contain at least one of the following important characteristics relative to the intended audience: proximity, prominence, timeliness, human interest, oddity, or consequence. The related term journalese is sometimes used, usually pejoratively, to refer to news-style writing. Another is headlinese. Overview Newspapers generally adhere to an expository writing style. Over time and place, journalism ethics and standards have varied in the degree of objectivity or sensationalism they incorporate. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |