|
I-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation-induced Cancer
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation (which lies on the boundary between ionizing and non-ionizing radiation). Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously or orally. Then, external detectors ( gamma cameras) capture and form images from the radiation emitted by the radiopharmaceuticals. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Iodine
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine. These are 123I, 124I, 125I, and 131I. All industrial production of radioactive iodine isotopes involves these four useful radionuclides. The isotope 135I has a half-life less than s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fukushima Daiichi Nuclear Disaster
The was a nuclear accident in 2011 at the Fukushima Daiichi Nuclear Power Plant in Ōkuma, Fukushima, Japan. The proximate cause of the disaster was the 2011 Tōhoku earthquake and tsunami, which occurred on the afternoon of 11 March 2011 and remains the most powerful earthquake ever recorded in Japan. The earthquake triggered a powerful tsunami, with 13–14-meter-high waves damaging the nuclear power plant's emergency diesel generators, leading to a loss of electric power. The result was the most severe nuclear accident since the Chernobyl disaster in 1986, classified as level seven on the International Nuclear Event Scale (INES) after initially being classified as level five, and thus joining Chernobyl as the only other accident to receive such classification. While the 1957 explosion at the Mayak facility was the second worst by radioactivity released, the INES ranks incidents by impact on population, so Chernobyl (335,000 people evacuated) and Fukushima (154,000 evacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydraulic Fracturing
Fracking (also known as hydraulic fracturing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of bedrock formations by a pressurized liquid. The process involves the high-pressure injection of "fracking fluid" (primarily water, containing sand or other proppants suspended with the aid of thickening agents) into a wellbore to create cracks in the deep-rock formations through which natural gas, petroleum, and brine will flow more freely. When the hydraulic pressure is removed from the well, small grains of hydraulic fracturing proppants (either sand or aluminium oxide) hold the fractures open. Hydraulic fracturing began as an experiment in 1947, and the first commercially successful application followed in 1950. As of 2012, 2.5 million "frac jobs" had been performed worldwide on oil and gas wells, over one million of those within the U.S. Such treatment is generally necessary to achieve adequate flow rates in shale gas, tight ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.Anderson, Don L.; "Chemical Composition of the Mantle" in ''Theory of the Earth'', pp. 147-175 Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graves' Disease
Graves' disease (german: Morbus Basedow), also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyroid. Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss. Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves' ophthalmopathy. About 25 to 30% of people with the condition develop eye problems. The exact cause of the disease is unclear; however, it is believed to involve a combination of genetic and environmental factors. A person is more likely to be affected if they have a family member with the disease. If one twin is affected, a 30% chance exists that the other twin will also have the disease. The onset of disease may b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-123
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV (this is the gamma primarily used for imaging). In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form. Production Iodine-123 is produced in a cyclotron by proton irradiation of xenon in a capsule. Xenon-124 absorbs a proton and immediately loses a neutron and proton to form xenon-123, or else loses two neutrons to form caesium-123, which decays to xenon-123. The xenon-123 formed by either route then decays to iodine-123, and is trapped on the inner wall of the irradiation capsule under refrigeration, then eluted with sodium hydroxide in a halogen disproportionation reaction, similar to col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Natural decay chain :\begin \ce \begin \ce \\ \ce \end \ce \\ \ce \begin \ce \\ \ce \end \ce \end Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/ mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
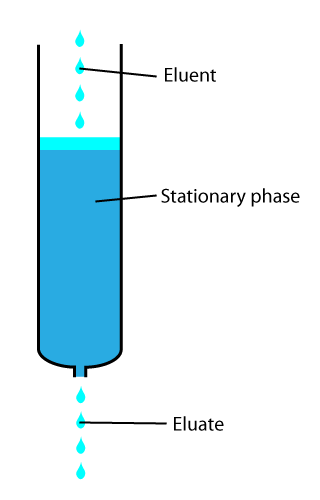
Eluted
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions. In a liquid chromatography experiment, for example, an analyte is generally adsorbed, or "bound to", an adsorbent in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Tellurium
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below. Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been found to be radioactive: 128Te and 130Te undergo double beta decay with half-lives of, respectively, 2.2×1024 (2.2 septillion) years (the longest half-life of all nuclides proven to be radioactive)Many isotopes are expected to have longer half-lives, but decay has not yet been observed in these, allowing only a lower limit to be placed on their half-lives and 8.2×1020 (820 quintillion) years. The longest-lived artificial radioisotope of tellurium is 121Te with a half-life of about 19 days. Several nuclear isomers have longer half-lives, the longest being 121mTe with a half-life of 154 days. The very-long-lived radioisotopes 128Te and 130Te are the two most common isotopes of tellurium. Of elements with at least one stable isotope, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)