|
Huntite
Huntite is a carbonate mineral with the chemical formula Mg3Ca(CO3)4. Huntite crystallizes in the trigonal system and typically occurs as platy crystals and powdery masses. For most of recorded history its main use was as a white pigment. Today the most common industrial use of huntite is as a natural mixture with hydromagnesite as a flame retardant or fire retardant additive for polymers. Discovery In 1953 a paper by George Faust announced the discovery of a new carbonate mineral found in Currant Creek, Nevada (US). Faust acknowledged that the mineral probably had been discovered previously, but it had been misidentified as impure magnesite by W. E. Ford in 1917. Faust named the new mineral "huntite" in honour of his former teacher, Walter Frederick Hunt (1882–1975), Professor of Petrology at the University of Michigan. Faust carried out analyses of the mineral, and found amongst others that in differential thermal analysis huntite showed two endothermic peaks, which could ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Hydroxide
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer Polymorphism (materials science), polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is Amphoterism, amphoteric, i.e., it has both Base (chemistry), basic and Acid (chemistry), acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water. Structure Al(OH)3 is built up of double layers of hydroxyl groups with aluminium ions occupying two-thirds of the octahedral holes between the two layers. Four Polymorphism (materials science), polymorphs are recognized. All feature layers of Octahedron, octahedral aluminium hydroxide units, with hydrogen bonds between the layers. The polymorphs differ in terms of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
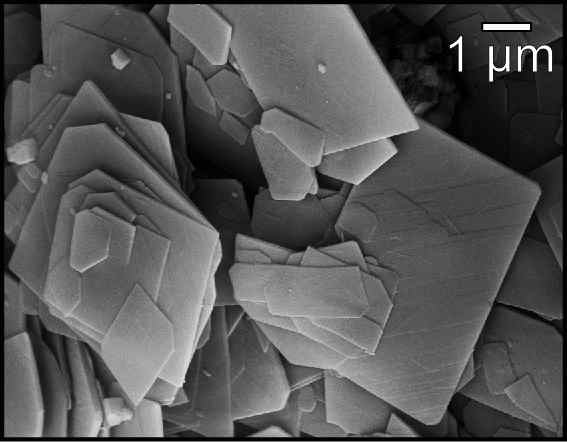
Hydromagnesite
Hydromagnesite is a hydrated magnesium carbonate mineral with the formula Mg5(CO3)4(OH)2·4H2O. It generally occurs associated with the weathering products of magnesium containing minerals such as serpentine or brucite. It occurs as incrustations and vein or fracture fillings in ultramafic rocks and serpentinites, and occurs in hydrothermally altered dolomite and marble. Hydromagnesite commonly appears in caves as speleothems and " moonmilk", deposited from water that has seeped through magnesium rich rocks. It is the most common cave carbonate after calcite and aragonite. The mineral thermally decomposes, over a temperature range of approximately 220 °C to 550 °C, releasing water and carbon dioxide leaving a magnesium oxide residue. Hydromagnesite was first described in 1836 for an occurrence in Hoboken, New Jersey. Stromatolites in an alkaline ( pH greater than 9) freshwater lake ( Salda Gölü) in southern Turkey are made of hydromagnesite precipitated by diatom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Retardant
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and are intended to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or (particularly for textiles) applied as a topical finish. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive. Classes Both Reactive and Additive Flame retardants types, can be further separated into four distinct classes: * Minerals such as aluminium hydroxide (ATH), magnesium hydroxide (MDH), huntite and hydromagnesite, various hydrates, red phosphorus, and boron compounds, mostly borates. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object. Fire retardants are commonly used in fire fighting, where they may be applied aerially or from the ground. Principles of operation In general, fire retardants reduce the flammability of materials by either blocking the fire physically or by initiating a chemical reaction that stops the fire. Physical action There are several ways in which the combustion process can be retarded by physical action: * By cooling: Some chemical reactions actually cool the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal ** Calcite CaCO3 ** Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 ** Otavite CdCO3 ** Rhodochrosite MnCO3 ** Siderite FeCO3 ** Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic ** Aragonite CaCO3 ** Cerussite PbCO3 ** Strontianite SrCO3 ** Witherite BaCO3 ** Rutherfordine UO2CO3 ** Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal ** Ankerite CaFe(CO3)2 ** Dolomite CaMg(CO3)2 ** Huntite Mg3Ca(CO3)4 ** Minrecordite CaZn(CO3)2 ** Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 ** Hydrocerussite Pb3(CO3)2(OH)2 ** Malachite Cu2CO3(OH)2 ** Rosasite (Cu,Zn)2CO3(OH)2 ** Phosgenite Pb2(CO3)Cl2 ** Hydrozincite Zn5(CO3)2(OH)6 ** Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates * Hydromagnesite Mg5(CO3)4(OH)2.4H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal ** Calcite CaCO3 ** Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 ** Otavite CdCO3 ** Rhodochrosite MnCO3 ** Siderite FeCO3 ** Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic ** Aragonite CaCO3 ** Cerussite PbCO3 ** Strontianite SrCO3 ** Witherite BaCO3 ** Rutherfordine UO2CO3 ** Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal ** Ankerite CaFe(CO3)2 ** Dolomite CaMg(CO3)2 ** Huntite Mg3Ca(CO3)4 ** Minrecordite CaZn(CO3)2 ** Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 ** Hydrocerussite Pb3(CO3)2(OH)2 ** Malachite Cu2CO3(OH)2 ** Rosasite (Cu,Zn)2CO3(OH)2 ** Phosgenite Pb2(CO3)Cl2 ** Hydrozincite Zn5(CO3)2(OH)6 ** Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates * Hydromagnesite Mg5(CO3)4(OH)2.4H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Sericytochromatia, the proposed name of the paraphyletic and most basal group, is the ancestor of both the non-photosynthetic group Melainabacteria and the photosynthetic cyanobacteria, also called Oxyphotobacteria. Cyanobacteria use photosynthetic pigments, such as carotenoids, phycobilins, and various forms of chlorophyll, which absorb energy from light. Unlike heterotrophic prokaryotes, cyanobacteria have internal membranes. These are flattened sacs called thylakoids where photosynthesis is performed. Phototrophic eukaryotes such as green plants perform photosynthesis in plasti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nesquehonite
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals. Forms The most common magnesium carbonate forms are the anhydrous salt called magnesite (), and the di, tri, and pentahydrates known as barringtonite (), nesquehonite (), and lansfordite (), respectively. Some basic forms such as artinite (), hydromagnesite (), and dypingite () also occur as minerals. All of those minerals are colouress or white. Magnesite consists of colourless or white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react with acids. Magnesite crystallizes in the calcite structure wherein is surrounded by six oxygen atoms. The dihydrate has a triclinic structure, while the trihydrate has a monoclinic structure. References to "light" and "heavy" magnesium carbonates actually refer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. 617. In such a process, a closed system usually absorbs thermal energy from its Environment (systems), surroundings, which is heat transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. It may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The term was coined by 19th-century French chemist Marcellin Berthelot. The opposite of an endothermic process is an exothermic process, one that releases or "gives out" energy, usually in the form of heat and sometimes as electrical energy. Thus in each term (endothermic and exothermic) the prefix refers to whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serpentinite
Serpentinite is a rock composed predominantly of one or more serpentine group minerals, the name originating from the similarity of the texture of the rock to that of the skin of a snake. Serpentinite has been called ''serpentine'' or ''serpentine rock'', particularly in older geological texts and in wider cultural settings.California Government Code § 425.2; ''see'' Formation and mineralogy Serpentinite is formed by near to complete serpentinization of mafic to ultramafic rocks. Serpentinite can be formed wherever ultramafic rock is infiltrated by water poor in carbon dioxide. This occurs at mid-ocean ridges and in the forearc mantle of subduction zones. The final mineral composition of serpentinite is usually dominated by lizardite, chrysotile, and magnetite. Brucite and antigorite are less commonly present. Lizardite, chrysotile, and antigorite all have approximately the formula or , but differ in minor components and in form. Accessory minerals, present in sm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)





