|
Hoechst 33342
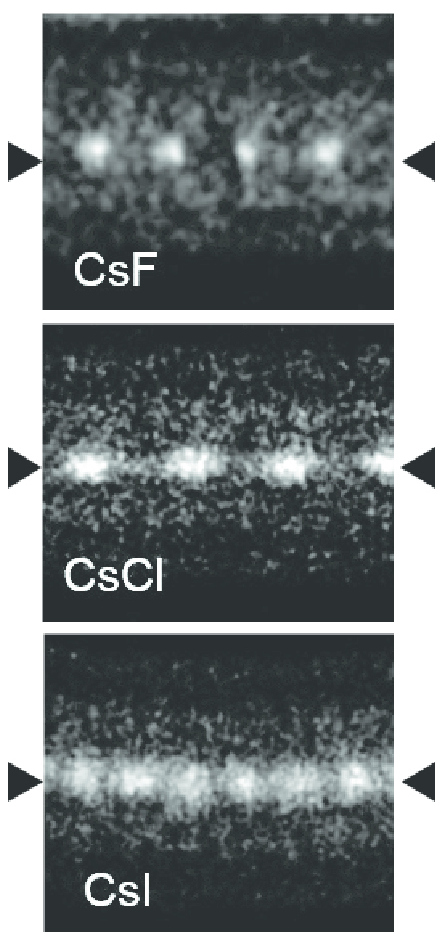
Bisbenzimide (Hoechst 33342) is an organic compound used as a fluorescent stain for DNA in molecular biology applications. Several related chemical compounds are used for similar purposes and are collectively called Hoechst stains. Application Bisbenzimide tends to bind to adenine–thymine-rich regions of DNA and can decrease its density. Bisbenzimide mixed with DNA samples can then be used to separate DNA according to their AT percentage using a cesium chloride Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ... (CsCl) gradient centrifugation. References {{reflist External links * Fluorescence Spectra: http://www.fluorophores.tugraz.at/substance/463 DNA-binding substances Fluorescent dyes Benzimidazoles Piperazines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Livin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoechst Stain
Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33,342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similar excitation–emission spectra. Molecular characteristics Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue-cyan fluorescent light around an emission spectrum maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510–540 nm range. Hoechst stains can be excited with a xenon- or mercury-arc lamp or with an ultraviolet laser. There is a considerable Stokes shift between the excitation and emission spectra that makes Hoechst dyes useful in experiments in which multiple fluorophores a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenine
Adenine () (symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its derivatives have a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate (ATP) and the cofactors nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD) and Coenzyme A. It also has functions in protein synthesis and as a chemical component of DNA and RNA. The shape of adenine is complementary to either thymine in DNA or uracil in RNA. The adjacent image shows pure adenine, as an independent molecule. When connected into DNA, a covalent bond is formed between deoxyribose sugar and the bottom left nitrogen (thereby removing the existing hydrogen atom). The remaining structure is called an ''adenine residue'', as part of a larger molecule. Adenosine is adenine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymine
Thymine () (symbol T or Thy) is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. In RNA, thymine is replaced by the nucleobase uracil. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calf thymus glands, hence its name. Derivation As its alternate name (5-methyluracil) suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thereby stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate), dTDP, or dTTP (for the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cesium Chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite. Caesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine applications such as treatment of cancer and diagnosis of myocardial in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoechst 33342 Stain - Platynereis Dumerilii Larvae
Hoechst, Hochst, or Höchst may refer to: * Hoechst AG, a former German life-sciences company * Hoechst stain, one of a family of fluorescent DNA-binding compounds * Höchst (Frankfurt am Main), a city district of Frankfurt am Main, Germany ** Frankfurt Höchst station, its railway station ** SG 01 Hoechst, German association football club * Höchst im Odenwald, a community in Hesse, Germany * Höchst, Austria, a municipality in Vorarlberg, Austria * Nicole Höchst (born 1970), German politician for the Alternative for Germany *''Hochst.'' taxonomic author abbreviation of Christian Ferdinand Friedrich Hochstetter (1787–1860), German botanist * Battle of Höchst (1622), fought between Catholic and Protestant armies * Battle of Höchst (1795) At the Battle of Höchst (11–12 October 1795), the Habsburg Austrian army commanded by François Sébastien Charles Joseph de Croix, Count of Clerfayt outmaneuvered the French Republican Army of Sambre-et-Meuse commanded by Jean-Bapti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Dyes
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. From an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzimidazoles
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a colorless solid. Preparation Benzimidazole is produced by condensation of o-phenylenediamine with formic acid,. or the equivalent trimethyl orthoformate: :C6H4(NH2)2 + HC(OCH3)3 → C6H4N(NH)CH + 3 CH3OH 2-substituted derivatives are obtained when the condensation is conducted with aldehydes in place of formic acid, followed by oxidation.Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Reactions Benzimidazole is a base: :C6H4N(NH)CH + H+ → 6H4(NH)2CHsup>+ It can also be deprotonated with stronger bases: :C6H4N(NH)CH + LiH → Li 6H4N2CH + H2 The imine can be alkylated and also serves as a ligand in coordination chemistry. The most prominent benzimidazole complex features ''N''-ribosyl-dimethylbenzimidazole as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |