|
Hyperdeformation
In nuclear physics, hyperdeformation is theoretically predicted states of an atomic nucleus with extremely elongated shape and very high angular momentum. Less elongated states, superdeformation, have been well observed, but the experimental evidence for hyperdeformation is more limited. Hyperdeformed states correspond to an axis ratio of 3:1. They would be caused by a third minimum in the potential energy surface, the second causing superdeformation and the first minimum being normal deformation. arXiv arXiv (pronounced "archive"—the X represents the Greek letter chi ⟨χ⟩) is an open-access repository of electronic preprints and postprints (known as e-prints) approved for posting after moderation, but not peer review. It consists of ...0902.0095v1/ref> Hyperdeformation is predicted to be found in 107Cd. References Nuclear physics {{nuclear-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superdeformation
In nuclear physics a superdeformed Atomic nucleus, nucleus is a nucleus that is very far from sphere, spherical, forming an ellipsoid with axes in ratios of approximately 2:1:1. Normal deformation is approximately 1.3:1:1. Only some nuclei can exist in superdeformed states. The first superdeformed states to be observed were the nuclear isomer, fission isomers, low-spin states of elements in the actinide and lanthanide series. The strong force decays much faster than the Coulomb force, which becomes stronger when nucleons are greater than 2.5 femtometers apart. For this reason, these elements undergo spontaneous fission. In the late 1980s, high-spin superdeformed rotational bands were observed in other regions of the periodic table. Specific elements include ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, gold, and mercury (element), mercury. The existence of superdeformed states occurs because of a combination of macroscopic and microscopic factors, which together ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to nuclear technology, applications in many fields. This includes nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the nucleosynthesis, origin of the chemical elements. History The history o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium atomic r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
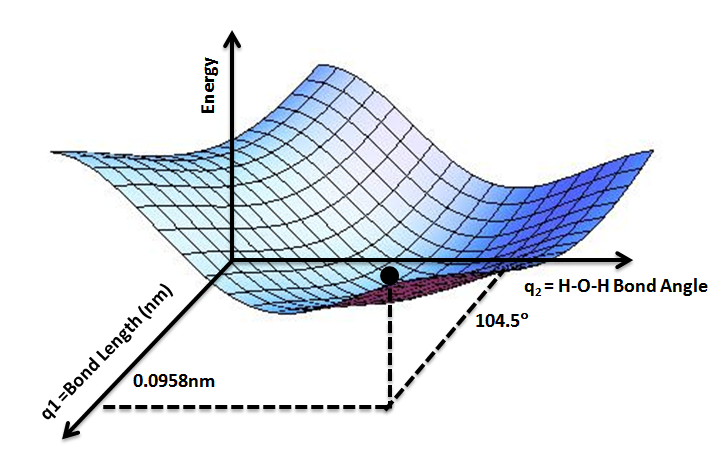
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ArXiv
arXiv (pronounced "archive"—the X represents the Greek letter chi ⟨χ⟩) is an open-access repository of electronic preprints and postprints (known as e-prints) approved for posting after moderation, but not peer review. It consists of scientific papers in the fields of mathematics, physics, astronomy, electrical engineering, computer science, quantitative biology, statistics, mathematical finance and economics, which can be accessed online. In many fields of mathematics and physics, almost all scientific papers are self-archived on the arXiv repository before publication in a peer-reviewed journal. Some publishers also grant permission for authors to archive the peer-reviewed postprint. Begun on August 14, 1991, arXiv.org passed the half-million-article milestone on October 3, 2008, and had hit a million by the end of 2014. As of April 2021, the submission rate is about 16,000 articles per month. History arXiv was made possible by the compact TeX file forma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Cadmium
Naturally occurring cadmium (48Cd) is composed of 8 isotopes. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decays have not been observed, due to extremely long half-lives. The two natural radioactive isotopes are 113Cd (beta decay, half-life is 8.04 × 1015 years) and 116Cd (two-neutrino double beta decay, half-life is 2.8 × 1019 years). The other three are 106Cd, 108Cd (double electron capture), and 114Cd (double beta decay); only lower limits on their half-life times have been set. At least three isotopes—110Cd, 111Cd, and 112Cd—are absolutely stable (except, theoretically, to spontaneous fission). Among the isotopes absent in natural cadmium, the most long-lived are 109Cd with a half-life of 462.6 days, and 115Cd with a half-life of 53.46 hours. All of the remaining radioactive isotopes have half-lives that are less than 2.5 hours and the majority of these have half-lives that are less than 5 minutes. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |