|
Huemulite
Huemulite is a mineral with formula Na4Mg(V10O28)·24H2O that is yellow to orange in color. It was first discovered in Argentina in 1959 and described in 1966. The mineral is named for the Huemul mine in which it was discovered. Occurrence and properties Huemulite is yellowish orange to orange and bright in color. The mineral occurs as thin films, aggregates of fine fibers, botryoidal masses, and interstitial filling in host sandstone. Huemulite has been found in Argentina, the Czech Republic, the United Kingdom, and the United States. It occurs in association with epsomite, gypsum, hummerite, rossite, and thenardite. Huemulite is a member of the pascoite group. It melts at to become a red liquid. Huemulite dissolves readily in cold water to produce an orange-yellow solution with a pH of 5.5 to 6.5.Gordillo, p. 12. Formation and synthesis In Argentina, the mineral formed after mine tunnels were cut through Cu- U deposits in sandstone and conglomerate.Gordillo, p. 3. The va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately. Simple oxides: *XO **Periclase group ***Periclase ***Manganosite **Zincite group ***Zincite *** Bromellite ***Tenorite ***Litharge * **Cuprite **Ice * **Hematite group ***Corundum ***Hematite ***Ilmenite * **Rutile group ***Rutile ***Pyrolusite ***Cassiterite **Baddeleyite **Uraninite **Thorianite * **Spinel group ***Spinel ***Gahnite ***Magnetite ***Franklinite ***Chromite **Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite **Manganite ** Romanèchite **Goethite group: ***Diaspore ***Goethite Nickel–Strunz Classification -04- Oxides IMA-CNMNC proposes a new hierarchical scheme (Mills et al., 2009). This list uses it to modify ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combustion. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
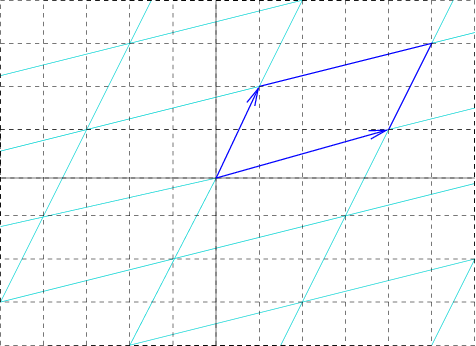
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula Unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. Examples include ionic and and covalent networks such as and C (as diamond or graphite).Steven S. Zumdahl; Susan A. Zumdahl (2000), ''Chemistry'' (5 ed.), Houghton Mifflin, pp. 470-6, Ionic compounds do not exist as individual molecules; a formula unit thus indicates the lowest reduced ratio of ions in the compound. In mineralogy, as minerals are almost exclusively either ionic or network solids, the formula unit is used. The number of formula units (Z) and the dimensions of the crystallographic axes are used in defining the unit cell In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal System
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family. The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (albeit there are many exceptions). Classifications Crystals can be classified in three ways: lattice systems, crystal systems and crystal families. The various classifications are often confused: in particular the trigonal crystal system is often confused with the rhombohedral lattice system, and the term "crystal system" is sometimes used to mean "latti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triclinic
180px, Triclinic (a ≠ b ≠ c and α ≠ β ≠ γ ) In crystallography, the triclinic (or anorthic) crystal system is one of the 7 crystal systems. A crystal system is described by three basis vectors. In the triclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. In addition, the angles between these vectors must all be different and may not include 90°. The triclinic lattice is the least symmetric of the 14 three-dimensional Bravais lattices. It has (itself) the minimum symmetry all lattices have: points of inversion at each lattice point and at 7 more points for each lattice point: at the midpoints of the edges and the faces, and at the center points. It is the only lattice type that itself has no mirror planes. Crystal classes The triclinic crystal system class names, examples, Schönflies notation, Hermann-Mauguin notation, point groups, International Tables for Crystallography space group number, orbifold, type, and sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Habit
In mineralogy, crystal habit is the characteristic external shape of an individual crystal or crystal group. The habit of a crystal is dependent on its crystallographic form and growth conditions, which generally creates irregularities due to limited space in the crystallizing medium (commonly in rocks).Klein, Cornelis, 2007, ''Minerals and Rocks: Exercises in Crystal and Mineral Chemistry, Crystallography, X-ray Powder Diffraction, Mineral and Rock Identification, and Ore Mineralogy,'' Wiley, third edition, Wenk, Hans-Rudolph and Andrei Bulakh, 2004, ''Minerals: Their Constitution and Origin,'' Cambridge, first edition, Recognizing the habit can aid in mineral identification and description, as the crystal habit is an external representation of the internal ordered atomic arrangement. Most natural crystals, however, do not display ideal habits and are commonly malformed. Hence, it is also important to describe the quality of the shape of a mineral specimen: * Euhedral: a cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acicular (crystal Habit)
__NOTOC__ Acicular, in mineralogy, refers to a crystal habit composed of slender, needle-like crystals. Crystals with this habit tend to be fragile. Complete, undamaged acicular specimens are uncommon. The term "acicular" derives from the Late Latin "acicula" meaning "little needle". Strictly speaking, the word refers to a growth habit that is slender and tapering to a point. Prismatic crystals are not acicular; however, colloquial usage has altered the commonly understood meaning of the word. When writing for mineralogical publications, authors should restrict their usage of "acicular" to crystals with the tapering growth habit. To add to the confusion, some minerals are described with various morphological terms. For example, natrolite is often described as slender prismatic and millerite is often described as filiform or capillary. Examples Minerals with an acicular habit include mesolite, natrolite, malachite, gypsum, rutile, brochantite and bultfonteinite. Crystals of dim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recrystallization (chemistry)
In chemistry, recrystallization is a technique used to purify chemicals. By dissolving a mixture of a compound and impurities in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. It is named for the crystals often formed when the compound precipitates out. Alternatively, ''recrystallization'' can refer to the natural growth of larger ice crystals at the expense of smaller ones. Chemistry In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted (see Separation process), recrystallization being one of them. There are also different recrystallization techniques that can be used such as: Single-solvent recrystallization Typically, the mixture of "compound A" and "impurity B" is dissolved in the smallest amount of hot solv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadic Acid
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes and are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively. A simple vanadate ion is the tetrahedral orthovanadate anion, (which is also called vanadate(V)), which is present in e.g. sodium orthovanadate and in solutions of in strong base ( pH > 13). Conventionally this ion is represented with a single double bond, however this is a resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms. Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions. There are also vanadates, such as rhodium vanadate, , which has a statistical rutile structure where the and ions randomly occupy the positions in the rutile lattice, that do not contain a lattice of cations and balanci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |