|
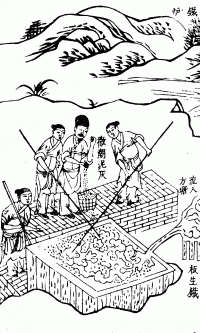
Hot-dip Galvanizing
Hot-dip galvanization is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around . When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (Oxygen, O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2, CO2) to form zinc carbonate (Smithsonite, ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances. Galvanized steel is widely used in applications where corrosion resistance is needed without the cost of stainless steel, and is considered superior in terms of cost and life-cycle. It can be identified by the crystallization patterning on the surface (often called a "spangle"). Galvanized steel can be welded; however, one must exercise caution around the resulting toxic zinc fumes. Galvanized fumes are released when the gal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Causticity
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction. Etymology The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', indicating how these substances seem to "gnaw" their way through flesh or other materials. Chemical terms The word ''corrosive'' refers to any chemical that will dissolve the structure of an object. They can be acids, oxidizers, or bases. When they come in contact with a surface, the surface deteriorates. The deterioration can happen in minutes, e.g. concentrated hydrochloric acid spilled on skin; or slowly over days or years, e.g. the rusting of iron in a bridge. Sometimes the word ''caustic'' is used as a synonym for ''corrosive'' when referring to the effect on living tissues. At low concentrations, a corrosive substance is called an ''irritant'', and its effect on living tissue is called irritation. At high concentrations, a corro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luigi Galvani
Luigi Galvani (, also ; ; la, Aloysius Galvanus; 9 September 1737 – 4 December 1798) was an Italian physician, physicist, biologist and philosopher, who studied animal electricity. In 1780, he discovered that the muscles of dead frogs' legs twitched when struck by an electrical spark. This was an early study of bioelectricity, following experiments by John Walsh and Hugh Williamson. Early life Luigi Galvani was born to Domenico Galvani and Barbara Caterina Foschi, in Bologna, then part of the Papal States. Domenico was a goldsmith. Galvani then began taking an interest in the field of "medical electricity". This field emerged in the middle of the 18th century, following electrical researches and the discovery of the effects of electricity on the human body by scientists including Bertrand Bajon and Ramón M. Termeyer in the 1760s, and by John Walsh and Hugh Williamson in the 1770s. Galvani vs. Volta Alessandro Volta, a professor of experimental physics in the Unive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Jacques Malouin
Paul Jacques Malouin (27 June 1701 – 3 January 1778) was a French physician and chemist. Career Born in Caen, Malouin graduated in medicine in 1730 against the wishes of his father (a legal official from Caen) who had sent him to Paris to study law. He settled in Paris in 1734 and opened a medical practice which attracted patients from the aristocracy and royal family. With the help of Fontenelle, a distant relation, he entered the French Academy of Sciences in 1742 where his particular research interest was the application of chemistry to medicine. In 1745 he was appointed professor of chemistry at the Jardin du Roi. For nine consecutive years he studied the epidemics raging in Paris and recorded the results of his research in his ''Mémoires'' published by the Academy of Sciences between 1746 and 1754, linking epidemic diseases to air temperature. In 1753 Malouin began a formal association with the royal court when he bought from Lassone the position of ''médecin de la re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode (positive electrode) is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance. It is used to build up thickness on undersized or worn-out parts, or to manufacture metal plates with complex shape, a process called electroforming. It is used to deposit copper and other conductors in forming printe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wrought Iron
Wrought iron is an iron alloy with a very low carbon content (less than 0.08%) in contrast to that of cast iron (2.1% to 4%). It is a semi-fused mass of iron with fibrous slag Inclusion (mineral), inclusions (up to 2% by weight), which give it a wood-like "grain" that is visible when it is etched, rusted, or bent to structural failure, failure. Wrought iron is tough, malleable, ductile, corrosion resistant, and easily forge welding, forge welded, but is more difficult to welding, weld electrically. Before the development of effective methods of steelmaking and the availability of large quantities of steel, wrought iron was the most common form of malleable iron. It was given the name ''wrought'' because it was hammered, rolled, or otherwise worked while hot enough to expel molten slag. The modern functional equivalent of wrought iron is Carbon steel#Mild or low-carbon steel, mild steel, also called low-carbon steel. Neither wrought iron nor mild steel contain enough carbon to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bucket
A bucket is typically a watertight, vertical Cylinder (geometry), cylinder or Truncation (geometry), truncated Cone (geometry), cone or square, with an open top and a flat bottom, attached to a semicircular carrying handle (grip), handle called the ''Bail handle, bail''. A bucket is usually an open-top container. In contrast, a Pail (container), pail can have a top or lid and is a shipping container. In common usage, the two terms are often used interchangeably. Types and uses A number of bucket types exist, used for a variety of purposes. Though most of these are functional purposes, a number, including those constructed from precious metals, are used for ceremonial purposes. Common types of bucket and their adjoining purposes include: * Water buckets used to carry water * Household and garden buckets used for carrying liquids and granular products * Elaborate ceremonial or ritual buckets constructed of bronze, ivory or other materials, found in several ancient or medieval cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrugated Galvanised Iron
Corrugated galvanised iron or steel, colloquially corrugated iron (near universal), wriggly tin (taken from UK military slang), pailing (in Caribbean English), corrugated sheet metal (in North America) and occasionally abbreviated CGI is a building material composed of sheets of hot-dip galvanised mild steel, cold-rolled to produce a linear ridged pattern in them. Although it is still popularly called "iron" in the UK, the material used is actually steel (which is iron alloyed with carbon for strength, commonly 0.3% carbon), and only the surviving vintage sheets may actually be made up of 100% iron. The corrugations increase the bending strength of the sheet in the direction perpendicular to the corrugations, but not parallel to them, because the steel must be stretched to bend perpendicular to the corrugations. Normally each sheet is manufactured longer in its strong direction. CGI is lightweight and easily transported. It was and still is widely used especially in rural a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recycling
Recycling is the process of converting waste materials into new materials and objects. The recovery of energy from waste materials is often included in this concept. The recyclability of a material depends on its ability to reacquire the properties it had in its original state. It is an alternative to "conventional" waste disposal that can save material and help lower greenhouse gas emissions. It can also prevent the waste of potentially useful materials and reduce the consumption of fresh raw materials, reducing energy use, air pollution (from incineration) and water pollution (from landfilling). Recycling is a key component of modern waste reduction and is the third component of the "Reduce, Reuse, and Recycle" waste hierarchy. It promotes environmental sustainability by removing raw material input and redirecting waste output in the economic system. There are some ISO standards related to recycling, such as ISO 15270:2008 for plastics waste and ISO 14001:2015 for enviro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dross
Dross is a mass of solid impurities floating on a molten metal or dispersed in the metal, such as in wrought iron. It forms on the surface of low- melting-point metals such as tin, lead, zinc or aluminium or alloys by oxidation of the metal. For higher melting point metals and alloys such as steel and silver, oxidized impurities melt and float making them easy to pour off. With wrought iron, hammering and later rolling remove some dross. With tin and lead the dross can be removed by adding sodium hydroxide pellets, which dissolve the oxides and form a slag. If floating, dross can also be skimmed off. Dross, as a solid, is distinguished from slag, which is a liquid. Dross product is not entirely waste material; for example, aluminium dross can be recycled and is also used in secondary steelmaking for slag deoxidation. Etymology and usage The term ''dross'' derives from the Old English word ''dros'', meaning the scum produced when smelting metals (extracting them from their ore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Ammonium Chloride
Zinc ammonium chloride is the inorganic compound with the formula (NH4)2ZnCl4. It is the ammonium salt of tetrachlorozincate. It used as a flux in the process of hot-dip galvanizing. Uses Steel to be galvanized passes through an acidic cleaning process to remove iron oxide "mill scale". After this process, the surface of the steel is very active and oxide layers begin forming immediately upon exposure to the atmosphere. Zinc ammonium chloride flux in aqueous solution is applied to the steel to reduce any oxides that are formed and/or inhibit them from forming altogether. This allows the molten zinc in the proceeding galvanizing step to maximally adhere to and alloy with the surface of the steel. Precautions Zinc ammonium chloride is a Class 9 hazardous material (Miscellaneous) according to the U.S. DOT. References {{Ammonium salts ammonium chloride Tetrachlorozincates zinc Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |