|
Hopkins–Cole Reaction
The Hopkins-Cole reaction, also known as the glyoxylic acid reaction, is a chemical test used for detecting the presence of tryptophan in proteins. A protein solution is mixed with Hopkins Cole reagent, which consists of glyoxylic acid. Concentrated sulfuric acid is slowly added to form two layers. A purple ring appears between the two layers if the test is positive for tryptophan. Nitrites, chlorates, nitrates and excess chlorides prevent the reaction from occurring. The reaction was first reported by Frederick Gowland Hopkins and Sydney W. Cole in 1901, as part of their work on the first isolation of tryptophan itself. References Protein methods Chemical tests {{analytical-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Test
In chemistry, a chemical test is a qualitative property, qualitative or Quantitative property, quantitative procedure designed to identify, quantify, or characterise a chemical compound or substituent, chemical group. Purposes Chemical testing might have a variety of purposes, such as to: * Determine if, or verify that, the requirements of a specification, regulation, or contract are met * Decide if a new product development program is on track: Demonstrate proof of concept * Demonstrate the utility of a proposed patent * Determine the interactions of a sample with other known substances * Determine the composition of a sample * Provide Technical standard, standard data for other scientific, medical, and Quality assurance functions * Validate suitability for end-use * Provide a basis for Technical communication * Provide a technical means of comparison of several options * Provide evidence in legal proceedings Biochemical tests * Clinistrips quantitatively test for sugar in uri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
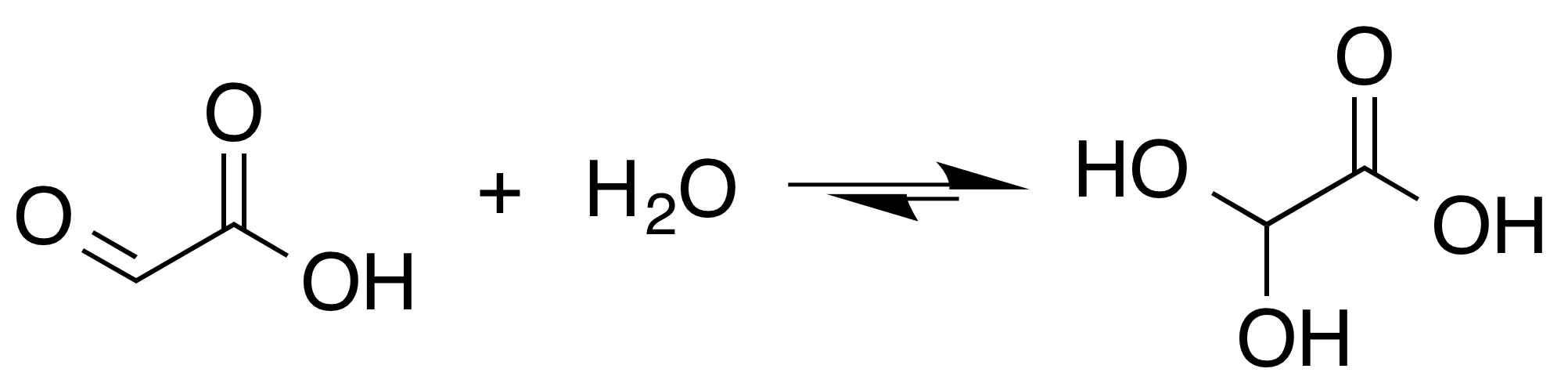
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ... with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Gowland Hopkins
Sir Frederick Gowland Hopkins (20 June 1861 – 16 May 1947) was an English biochemist who was awarded the Nobel Prize in Physiology or Medicine in 1929, with Christiaan Eijkman, for the discovery of vitamins, even though Casimir Funk, a Polish biochemist, is widely credited with discovering vitamins. He also discovered the amino acid tryptophan, in 1901. He was President of the Royal Society from 1930 to 1935. Education and early life Hopkins was born in Eastbourne, Sussex, and initially attended the City of London School. However, he soon transferred to Alexandra Park College in Hornsey and completed his further study with the University of London External Programme (through evening classes at Birkbeck College) and the medical school at Guy's Hospital which is now part of King's College London School of Medicine. Career and research After graduating, Hopkins then taught physiology and toxicology at Guy's Hospital from 1894 to 1898. In 1898, while attending a meeting of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sydney W
Sydney ( ) is the capital city of the States and territories of Australia, state of New South Wales, and the most populous city in both Australia and List of cities in Oceania by population, Oceania. Located on Australia's east coast, the metropolis surrounds Sydney Harbour and extends about towards the Blue Mountains (New South Wales), Blue Mountains to the west, City of Hawkesbury, Hawkesbury to the north, the Royal National Park to the south and Macarthur, New South Wales, Macarthur to the south-west. Sydney is made up of 658 suburbs, spread across 33 local government areas. Residents of the city are known as "Sydneysiders". The 2021 census recorded the population of Greater Sydney as 5,231,150, meaning the city is home to approximately 66% of the state's population. Estimated resident population, 30 June 2017. Nicknames of the city include the 'Emerald City' and the 'Harbour City'. Indigenous Australians, Aboriginal Australians have inhabited the Greater Sydney region for a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Methods
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins (e.g., for detecting proteins, for isolating and purifying proteins, and for characterizing the structure and function of proteins, often requiring that the protein first be purified). Computational methods typically use computer programs to analyze proteins. However, many experimental methods (e.g., mass spectrometry) require computational analysis of the raw data. Genetic methods Experimental analysis of proteins typically requires expression and purification of proteins. Expression is achieved by manipulating DNA that encodes the protein(s) of interest. Hence, protein analysis usually requires DNA methods, especially cloning. Some examples of genetic methods include conceptual translation, Site-directed mutagenesis, using a fusion protein, and matching allele with disease states. Some proteins have never been directly sequenced, however by translating codons from know ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |