|
Hexahydroxybenzene Triscarbonate
Hexahydroxybenzene triscarbonate is a chemical compound, an oxide of carbon with formula . Its molecular structure consists of a benzene core with the six hydrogen atoms replaced by three carbonate groups. It can be seen as a sixfold ester of hexahydroxybenzene (benzenehexol) and carbonic acid. The compound was obtained by C. Nallaiah in 1984, as a tetrahydrofuran solvate Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the .... C. Nallaiah (1984), ''Synthesis of tetrahydroxy-1,4-benzoquinone biscarbonate and hexahydroxybenzene triscarbonate - new organic carbon oxides''Tetrahedron, Volume 40, Issue 23, 1984, Pages 4897-4900 See also * Tetrahydroxy-1,4-benzoquinone biscarbonate * Tetrahydroxy-1,4-benzoquinone bisoxalate * Hexahydroxybenzene trisoxalate References {{Oxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxocarbon
In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (). Many other stable (practically if not thermodynamically) or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide ( or ) and mellitic anhydride (). Many other oxides are known today, most of them synthesized since the 1960s. Some of these new oxides are stable at room temperature. Some are metastable or stable only at very low temperatures, but decompose to simpler oxocarbons when warmed. Many are inherently unstable and can be observed only momentarily as intermediates in chemical reactions or are so reactive that they exist only in gas phase or have only been detected by matrix isolation. Graphene oxide and other stable polymeric carbon oxides with unbounded molecular structures exist. Overview Carbon dioxide (CO2) occurs widely in nature, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
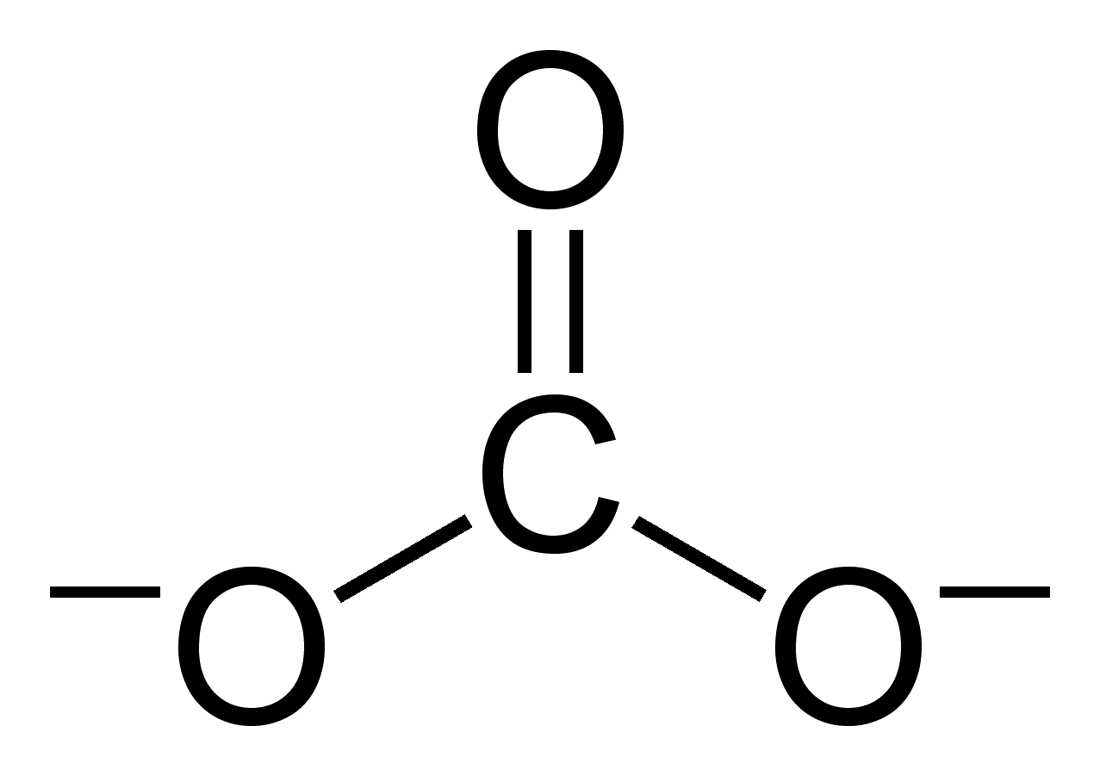
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahydroxybenzene
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula or . It is a six-fold phenol of benzene. The product is also called hexaphenol, but this name has been used also for other substances. Benzenehexol is a crystalline solid soluble in hot water, with a melting point above 310°. It can be prepared from inositol (cyclohexanehexol). Oxidation of benzenehexol yields tetrahydroxy-''p''-benzoquinone (THBQ), rhodizonic acid, and dodecahydroxycyclohexane. Conversely, benzenehexol can be obtained by reduction of sodium THBQ salt with SnCl2/HCl. Benzenehexol is a starting material for a class of discotic liquid crystals. Benzenehexol forms an adduct with 2,2'-bipyridine, with 1:2 molecular ratio. Benzenehexolate Like most phenols, benzenehexol can lose the six H+ ions from the hydroxyl groups, yielding the hexaanion . The potassium salt of this anion is one of the components of Liebig's so-called "potassium carbonyl", the product of the reaction of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvate
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration. Solubility of solid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxy-1,4-benzoquinone Biscarbonate
Tetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic acid. The compound was obtained by C. Nallaiah in 1984, as a tetrahydrofuran solvate Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the .... C. Nallaiah (1984), ''Synthesis of tetrahydroxy-1,4-benzoquinone biscarbonate and hexahydroxybenzene triscarbonate - new organic carbon oxides''Tetrahedron, Volume 40, Issue 23, 1984, Pages 4897-4900 See also * Tetrahydroxy-1,4-benzoquinone bisoxalate * Hexahydroxybenzene trisoxalate * Hexahydroxybenzene triscarbonate References {{Oxides of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxy-1,4-benzoquinone Bisoxalate
Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula . Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid. The compound was first described by H. S. Verter, H. Porter, and R. Dominic in 1968. It was obtained by reacting tetrahydroxy-1,4-benzoquinone with oxalyl chloride in tetrahydrofuran. It is a yellow solid that can be crystallized as a tetrahydrofuran solvate Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the ..., but could not be prepared in pure form. H. S. Verter, H. Porter, and R. Dominic (Verter, Porter and Dominic, 1968), ''A new carbon oxide: synthesis of tetrahydroxybenzoquinone bisox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)