|
Heavy Metal Detoxification
Heavy metal detox, or detoxification, is the removal of metallic toxic substances from the body. In conventional medicine, detoxification can also be achieved artificially by techniques such as dialysis and (in a very limited number of cases) chelation therapy. There is a firm scientific base in evidence-based medicine for this type of detoxification . Many alternative medicine practitioners promote various other types of detoxification such as "diet detoxification" . The detox is performed to rid the body of toxic metals. Toxic metals, including heavy metals, are individual metals and metal compounds that negatively affect people's health. Some toxic, semi-metallic elements, including arsenic and selenium, are discussed in this page. In very small amounts, many of these metals are necessary to support life. However, in larger amounts, they become toxic. They may build up in biological systems and become a significant health hazard. This page provides a starting point for tech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detoxification (alternative Medicine)
Detoxification (often shortened to detox and sometimes called body cleansing) is a type of alternative-medicine treatment which aims to rid the body of unspecified "toxins" – substances that proponents claim accumulate in the body over time and have undesirable short-term or long-term effects on individual health. Activities commonly associated with detoxification include dieting, fasting, consuming exclusively or avoiding specific foods (such as fats, carbohydrates, fruits, vegetables, juices, herbs), colon cleansing, chelation therapy, and the removal of dental fillings containing amalgam. Scientists and health organizations have criticized the concept of detoxification for its unsound scientific basis and for the lack of evidence for claims made. The "toxins" usually remain undefined, with little to no evidence of toxic accumulation in the patient. The British organisation Sense About Science has described some detox diets and commercial products as "a waste of time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solder
Solder (; NA: ) is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics. Soft solder typically has a melting point range of , and is commonly used in electronics, plumbing, and sheet metal work. Alloys that melt between are the most commonly used. Soldering performed using alloys with a melting point above is called "hard soldering", "silver soldering", or brazing. In specific proportions, some alloys are eutectic — that is, the alloy's melting point is the lowest possible for a mixture of those components, and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiator
Radiators are heat exchangers used to transfer thermal energy from one medium to another for the purpose of cooling and heating. The majority of radiators are constructed to function in cars, buildings, and electronics. A radiator is always a source of heat to its environment, although this may be for either the purpose of heating this environment, or for cooling the fluid or coolant supplied to it, as for automotive engine cooling and HVAC dry cooling towers. Despite the name, most radiators transfer the bulk of their heat via convection instead of thermal radiation. History The Roman hypocaust is an early example of a type of radiator for building space heating. Franz San Galli, a Prussian-born Russian businessman living in St. Petersburg, is credited with inventing the heating radiator around 1855, having received a radiator patent in 1857, but American Joseph Nason developed a primitive radiator in 1841 and received a number of U.S. patents for hot water and steam heating. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
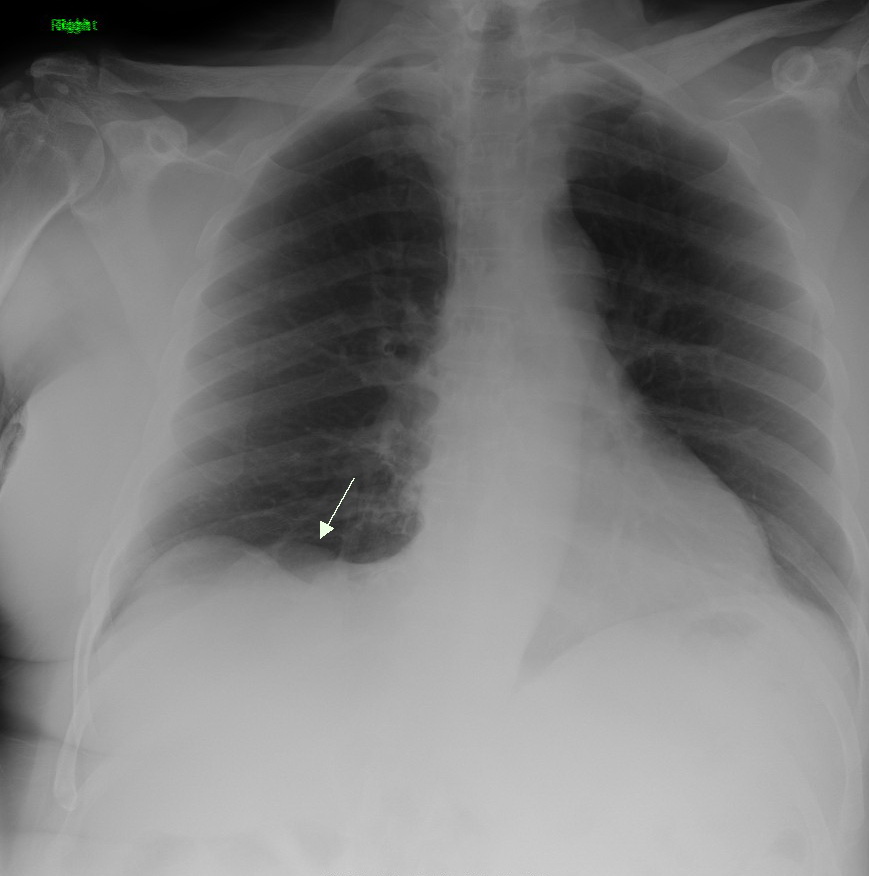
Lung Cancer
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissue (biology), tissues of the lung. Lung carcinomas derive from transformed, malignant cells that originate as epithelial cells, or from tissues composed of epithelial cells. Other lung cancers, such as the rare sarcomas of the lung, are generated by the malignant transformation of connective tissues (i.e. nerve, fat, muscle, bone), which arise from mesenchymal cells. Lymphomas and melanomas (from lymphoid and melanocyte cell lineages) can also rarely result in lung cancer. In time, this uncontrolled neoplasm, growth can metastasis, metastasize (spreading beyond the lung) either by direct extension, by entering the lymphatic circulation, or via hematogenous, bloodborne spread – into nearby tissue or other, more distant parts of the body. Most cancers that originate from within the lungs, known as primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Chromate
Zinc chromate, Zn Cr O4, is a chemical compound containing the chromate anion, appearing as odorless yellow powder or yellow-green crystals, but, when used for coatings, pigments are often added. It is used industrially in chromate conversion coatings, having been developed by the Ford Motor Company in the 1920s. Production A process known as the Cronak process is used to create zinc chromate for use in industry. This process is done by putting zinc or a zinc plated metal in a solution of sodium dichromate and sulfuric acid for a few seconds. Zinc chromate can also be synthesized by using neutral potassium chromate (K2CrO4) and zinc sulfate (ZnSO4), which forms a precipitate. K2CrO4 + ZnSO4 → ZnCrO4 + K2SO4 Uses Zinc chromate's main use is in industrial painting as a coating over iron or aluminum materials. It was used extensively on aircraft by the US military, especially during the 1930s and 1940s. It is also used in a variety of paint coatings for the aerospace and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Chromate
Strontium chromate is a chemical compound, with the formula SrCrO4. US National Institutes of Health TOXNET Preparation Strontium chromate is prepared from the reaction of with , or from a reaction between with . Reac ...
|
Lead Chromate
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: :H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. : The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Chromate
Calcium chromate is an inorganic compound with the formula CaCrO4, ''i.e.'' the chromate salt of calcium. It is a bright yellow solid which is normally found in the dihydrate form CaCrO4·2H2O. A very rare anhydrous mineral form exists in nature, which is known as chromatite. The compound is occasionally used as a pigment, but this usage is limited due to the very toxic and carcinogenic nature of hexavalent chromium compounds such as chromate salts. Synthesis and reactions Calcium chromate is formed from the salt metathesis reaction of sodium chromate and calcium chloride: :Na2CrO4 + CaCl2 → CaCrO4 + 2 NaCl In aqueous solution the dihydrate is obtained, which loses water to afford the anhydrate at 200 °C. It is an oxidiser, oxidising organic compounds (''e.g.'' alcohols) or reducing agents (''e.g.'' metals) to the corresponding carbonyl compounds or metal oxides while the chromium(VI) centre in CaCrO4 is reduced to chromium(III). Solid calcium chromate will react e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexavalent Chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the salt sodium dichromate. Hexavalent chromium is key to all materials made from chromium. Approximately of hexavalent chromium were produced in 1985. Additional hexavalent chromium compounds include chromium trioxide and various salts of chromate and dichromate, among others. Hexavalent chromium is used in textile dyes, wood preservation, anti-corrosion products, chromate conversion coatings, and a variety of niche uses. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anticorrosive agents to paints, primers, and other surface coatings; and chromic acid electroplated onto metal parts to provide a decorative or protective coating. Hexavalent chromium can be fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






-oxid.jpg)