|
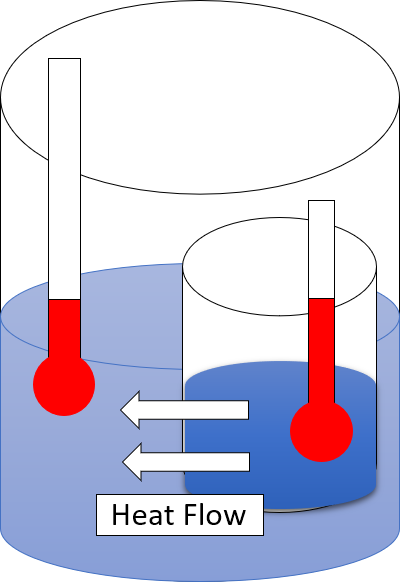
Heat Rejection
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glowing Metal
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Watt
The watt (symbol: W) is the unit of power or radiant flux in the International System of Units (SI), equal to 1 joule per second or 1 kg⋅m2⋅s−3. It is used to quantify the rate of energy transfer. The watt is named after James Watt (1736–1819), an 18th-century Scottish inventor, mechanical engineer, and chemist who improved the Newcomen engine with his own steam engine in 1776. Watt's invention was fundamental for the Industrial Revolution. Overview When an object's velocity is held constant at one metre per second against a constant opposing force of one newton, the rate at which work is done is one watt. : \mathrm In terms of electromagnetism, one watt is the rate at which electrical work is performed when a current of one ampere (A) flows across an electrical potential difference of one volt (V), meaning the watt is equivalent to the volt-ampere (the latter unit, however, is used for a different quantity from the real power of an electrical circuit). : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Mazur
Peter Mazur (born Vienna, Austria, 11 December 1922; died Lausanne, Switzerland, 15 August 2001) was an Austrian-born, Dutch physicist and one of the founders of the field of non-equilibrium thermodynamics. He is the father of Harvard University physics professor Eric Mazur. Family Peter Mazur was born on 11 December 1922 in Vienna, Austria. His father, Karl Georg Mazur, a businessman, and mother, Anna Zula Lecker, frequently moved during Mazur's youth. In 1931 the family left for Berlin, where Mazur attended the Franzősisches Gymnasium. Two years later the family left Germany to escape the growing threat of National Socialism. After spending one year in Switzerland they moved to Paris where Mazur attended the Lycée Janson de Sailly. In 1939 Mazur moved to The Hague in the Netherlands, but in 1940 the occupying Nazis no longer permitted Jews to live near the seacoast and the family moved to Zeist. In 1942 Mazur and his family went into hiding and he spent three years on various f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Production
Entropy production (or generation) is the amount of entropy which is produced in any irreversible processes such as heat and mass transfer processes including motion of bodies, heat exchange, fluid flow, substances expanding or mixing, anelastic deformation of solids, and any irreversible thermodynamic cycle, including thermal machines such as power plants, heat engines, refrigerators, heat pumps, and air conditioners. In the dual representation entropy–exergy for accounting the second law of thermodynamics it can be expressed in equivalent terms of exergy disruption. Short history Entropy is produced in irreversible processes. The importance of avoiding irreversible processes (hence reducing the entropy production) was recognized as early as 1824 by Carnot. In 1865 Rudolf Clausius expanded his previous work from 1854 on the concept of "unkompensierte Verwandlungen" (uncompensated transformations), which, in our modern nomenclature, would be called the entropy production. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exact Differential
In multivariate calculus, a differential or differential form is said to be exact or perfect (''exact differential''), as contrasted with an inexact differential, if it is equal to the general differential dQ for some differentiable function Q in an orthogonal coordinate system. An exact differential is sometimes also called a ''total differential'', or a ''full differential'', or, in the study of differential geometry, it is termed an exact form. The integral of an exact differential over any integral path is path-independent, and this fact is used to identify state functions in thermodynamics. Overview Definition Even if we work in three dimensions here, the definitions of exact differentials for other dimensions are structurally similar to the three dimensional definition. In three dimensions, a form of the type :A(x,y,z) \,dx + B(x,y,z) \,dy + C(x,y,z) \,dz is called a differential form. This form is called ''exact'' on an open domain D \subset \mathbb^3 in spac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inexact Differential
An inexact differential or imperfect differential is a differential whose integral is path dependent. It is most often used in thermodynamics to express changes in path dependent quantities such as heat and work, but is defined more generally within mathematics as a type of differential form. In contrast, an integral of an exact differential is always path independent since the integral acts to invert the differential operator. Consequently, a quantity with an inexact differential cannot be expressed as a function of only the variables within the differential. I.e., its value cannot be inferred just by looking at the initial and final states of a given system. Inexact differentials are primarily used in calculations involving heat and work because they are path functions, not state functions. Definition An inexact differential \delta u is a differential for which the integral over some two paths with the same end points is different. Specifically, there exist integrable paths ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
In thermodynamics, work is one of the principal processes by which a thermodynamic system can interact with its surroundings and exchange energy. An exchange of energy is facilitated by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings, or vice versa. In the surroundings, this mechanical work can lift a weight, for example. The externally measured forces and external effects may be electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . gravitational,North, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . or mechanical (such as pressure-v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Heat
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics. History Contributions from antiquity The ancients viewed heat as that related to fire. In 3000 BC, the ancient Egyptians viewed heat as related to origin mythologies. The ancient Indian philosophy in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
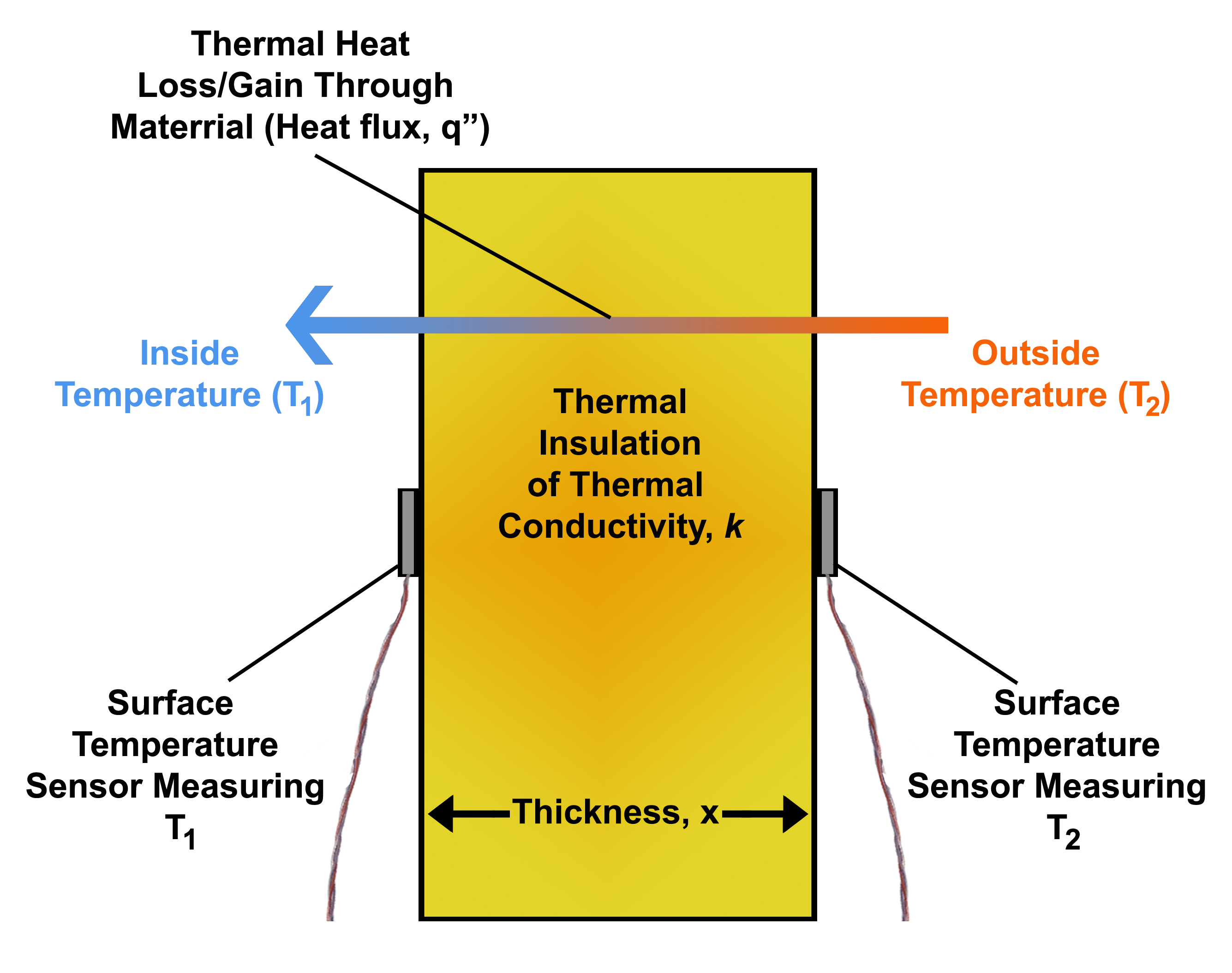
Heat Flux
Heat flux or thermal flux, sometimes also referred to as ''heat flux density'', heat-flow density or ''heat flow rate intensity'' is a flow of energy per unit area per unit time. In SI its units are watts per square metre (W/m2). It has both a direction and a magnitude, and so it is a vector quantity. To define the heat flux at a certain point in space, one takes the limiting case where the size of the surface becomes infinitesimally small. Heat flux is often denoted \vec_\mathrm, the subscript specifying ''heat'' flux, as opposed to ''mass'' or ''momentum'' flux. Fourier's law is an important application of these concepts. Fourier's law For most solids in usual conditions, heat is transported mainly by conduction and the heat flux is adequately described by Fourier's law. Fourier's law in one dimension \phi_\text = -k \frac where k is the thermal conductivity. The negative sign shows that heat flux moves from higher temperature regions to lower temperature regions. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)