|
Grp78
Binding immunoglobulin protein (BiP) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the ''HSPA5'' gene. BiP is a HSP70 molecular chaperone located in the lumen of the endoplasmic reticulum (ER) that binds newly synthesized proteins as they are translocated into the ER, and maintains them in a state competent for subsequent folding and oligomerization. BiP is also an essential component of the translocation machinery and plays a role in retrograde transport across the ER membrane of aberrant proteins destined for degradation by the proteasome. BiP is an abundant protein under all growth conditions, but its synthesis is markedly induced under conditions that lead to the accumulation of unfolded polypeptides in the ER. Structure BiP contains two functional domains: a nucleotide-binding domain (NBD) and a substrate-binding domain (SBD). The NBD binds and hydrolyzes ATP, and the SBD binds po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unfolded Protein Response
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum (ER) stress. It has been found to be conserved between all mammalian species, as well as yeast and worm organisms. The UPR is activated in response to an accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum. In this scenario, the UPR has three aims: initially to restore normal function of the cell by halting protein translation, degrading misfolded proteins, and activating the signalling pathways that lead to increasing the production of molecular chaperones involved in protein folding. If these objectives are not achieved within a certain time span or the disruption is prolonged, the UPR aims towards apoptosis. Sustained overactivation of the UPR has been implicated in prion diseases as well as several other neurodegenerative diseases, and inhibiting the UPR could become a treatment for those diseases. Diseases amenable to UPR inhibition inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HSP70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures. Discovery Members of the Hsp70 family are very strongly upregulated by heat stress and toxic chemicals, particularly heavy metals such as arsenic, cadmium, copper, mercury, etc. Heat shock was originally discovered by Ferruccio Ritossa in the 1960s when a lab worker accidentally boosted the incubation temperature of Drosophila (fruit flies). When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hsp70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures. Discovery Members of the Hsp70 family are very strongly upregulated by heat stress and toxic chemicals, particularly heavy metals such as arsenic, cadmium, copper, mercury, etc. Heat shock was originally discovered by Ferruccio Ritossa in the 1960s when a lab worker accidentally boosted the incubation temperature of Drosophila (fruit flies). When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
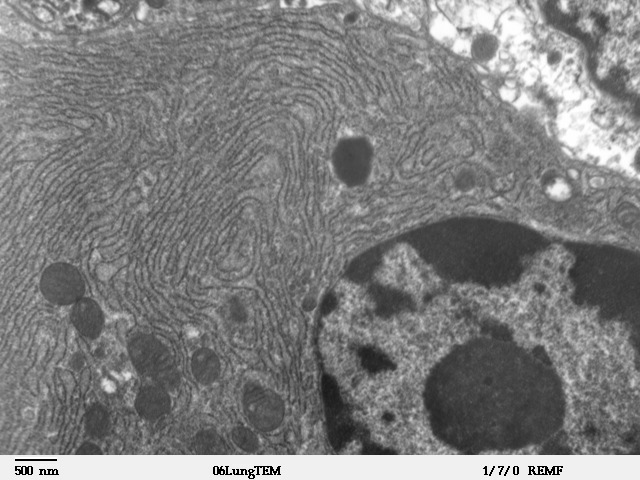
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose-regulated Protein
Glucose-regulated protein is a protein in the endoplasmic reticulum in the cell. It comes in several different molecular masses, including: *Grp78 (78 kDa) *Grp94 (94 kDa) *Grp170 (170 kDa), which is a human chaperone protein In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assi ... References Endoplasmic reticulum resident proteins {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permissive Temperature
Temperature-sensitive mutants are variants of genes that allow normal function of the organism at low temperatures, but altered function at higher temperatures. Cold sensitive mutants are variants of genes that allow normal function of the organism at higher temperatures, but altered function at low temperatures. Mechanism Most temperature-sensitive mutations affect proteins, and cause loss of protein function at the non-permissive temperature. The permissive temperature is one at which the protein typically can fold properly, or remain properly folded. At higher temperatures, the protein is unstable and ceases to function properly. These mutations are usually recessive in diploid organisms. Temperature sensitive mutants arrange a reversible mechanism and are able to reduce particular gene products at varying stages of growth and are easily done by changing the temperature of growth. Permissive temperature The permissive temperature is the temperature at which a temperature-sen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secretory Protein
A secretory protein is any protein, whether it be endocrine or exocrine, which is secreted by a cell. Secretory proteins include many hormones, enzymes, toxins, and antimicrobial peptides. Secretory proteins are synthesized in the endoplasmic reticulum. Production The production of a secretory protein starts like any other protein. The mRNA is produced and transported to the cytosol where it interacts with a free cytosolic ribosome. The part that is produced first, the N-terminal, contains a signal sequence consisting of 6 to 12 amino acids with hydrophobic side chains. This sequence is recognised by a cytosolic protein, SRP (Signal Recognition Particle), which stops the translation and aids in the transport of the mRNA-ribosome complex to an SRP receptor found in the membrane of the endoplasmic reticulum. When it arrives at the ER, the signal sequence is transferred to the translocon, a protein-conducting channel in the membrane that allows the newly synthesized polypeptide to b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Foldase
In molecular biology, foldases are a particular kind of molecular chaperones that assist the non-covalent folding of proteins in an ATP-dependent manner. Examples of foldase systems are the GroEL/GroES and the DnaK/DnaJ/GrpE system. References {{reflist http://www.embl.de/pepcore/pepcore_services/protein_expression/ecoli/improving_protein_solubility/ See also * Holdase * Chaperonin * Co-chaperone Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation ... Molecular chaperones Protein biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holdase
In molecular biology, holdases are a particular kind of molecular chaperones that assist the non-covalent folding of proteins in an ATP-independent manner. Examples of holdases are DnaJ and Hsp33. Holdases bind to protein folding intermediates to prevent their aggregation but without directly refolding them. They stand in opposition to foldases, which are chaperones that use ATP to fold proteins. See also * Foldase * Chaperonin * Co-chaperone Co-chaperones are proteins that assist chaperone (protein), chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly esse ... References {{reflist Molecular chaperones Protein biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Invertase
Invertase is an enzyme that catalyzes the hydrolysis (breakdown) of sucrose (table sugar) into fructose and glucose. Alternative names for invertase include , saccharase, glucosucrase, beta-h-fructosidase, beta-fructosidase, invertin, sucrase, maxinvert L 1000, fructosylinvertase, alkaline invertase, acid invertase, and the systematic name: beta-fructofuranosidase. The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose. Invertase is a glycoprotein that hydrolyses (cleaves) the non-reducing terminal beta-fructofuranoside residues. Thus, its systematic name is beta-fructofuranosidase. Invertases cleave the O-C(fructose) bond, whereas the sucrases cleave the O-C(glucose) bond. Invertase cleaves the alpha-1,2-glycosidic bond of sucrose. For industrial use, invertase is usually derived from yeast. It is also synthesized by bees, which us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Factor
The α-factor is used to predict the solid–liquid interface type of a material during solidification. Method According to John E. Gruzleski in his book Microstructure Development During Metalcasting (1996): α = (L/k*TE)*(η/v) where L is latent heat of fusion k is Boltzmann’s constant TE is equilibrium freezing temperature η is the number of nearest neighbours an atom has in the interface plane v is the number of nearest neighbours in the bulk solid Since L/TE = ΔSf where ΔSf is the molar entropy of fusion of the material α = ΔSf/k(η/v) According to Martin Glicksman in his book Principles of Solidification : An Introduction to Modern Casting and Crystal Growth Concepts(2011): α = ΔSf/Rg(η1/Z) where Rg is the universal gas constant The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |