|
Glycogen Phosphorylase
Glycogen phosphorylase is one of the phosphorylase enzymes (). Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects. Mechanism Glycogen phosphorylase breaks up glycogen into glucose subunits (see also figure below): (α-1,4 glycogen chain)n + Pi ⇌ (α-1,4 glycogen chain)n-1 + α-D-glucose-1-phosphate. Glycogen is left with one fewer glucose molecule, and the free glucose molecule is in the form of glucose-1-phosphate. In order to be used for metabolism, it must be converted to glucose-6-phosphate by the enzyme phosphoglucomutase. Although the reaction is reversible in vitro, within the cell the enzyme only works in the forward direction as shown below because the concentration of inorganic phosphate is much higher than that of glucose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylase
In biochemistry, phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate (phosphate+hydrogen) to an acceptor. :A-B + P A + P-B They include allosteric enzymes that catalyze the production of glucose-1-phosphate from a glucan such as glycogen, starch or maltodextrin. Phosphorylase is also a common name used for glycogen phosphorylase in honor of Earl W. Sutherland Jr., who in the late 1930s discovered it as the first phosphorylase. Function Phosphorylases should not be confused with phosphatases, which remove phosphate groups. In more general terms, phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate (phosphate + hydrogen) to an acceptor, not to be confused with a phosphatase (a hydrolase) or a kinase (a phosphotransferase). A phosphatase removes a phosphate group from a donor using water, whereas a kinase transfers a phosphate group from a donor (usually ATP) to an acceptor. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Branching (polymer Chemistry)
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent (e.g. a hydrogen atom) on a monomer subunit by another covalently-bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type. Branched polymers have more compact and symmetrical molecular conformations, and exhibit intra-heterogeneous dynamical behavior with respect to the unbranched polymers. In crosslinking rubber by vulcanization, short sulfur branches link polyisoprene chains (or a synthetic variant) into a multiple-branched thermosetting elastomer. Rubber can also be so completely vulcanized that it becomes a rigid solid, so hard it can be used as the bit in a smoking pipe. Polycarbonate chains can be crosslinked to form the hardest, most impact-resistant thermosetting plastic, used in safety glasses. Branching may result from the formation of carbon-carbon or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isozyme
In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. different ''K''M values), or are regulated differently. They permit the fine-tuning of metabolism to meet the particular needs of a given tissue or developmental stage. In many cases, isozymes are encoded by homologous genes that have diverged over time. Strictly speaking, enzymes with different amino acid sequences that catalyse the same reaction are isozymes if encoded by different genes, or allozymes if encoded by different alleles of the same gene; the two terms are often used interchangeably. Introduction Isozymes were first described by R. L. Hunter and Clement Markert (1957) who defined them as ''different variants of the same enzyme having identical functions and present in the same individual''. This definition encompasses (1) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
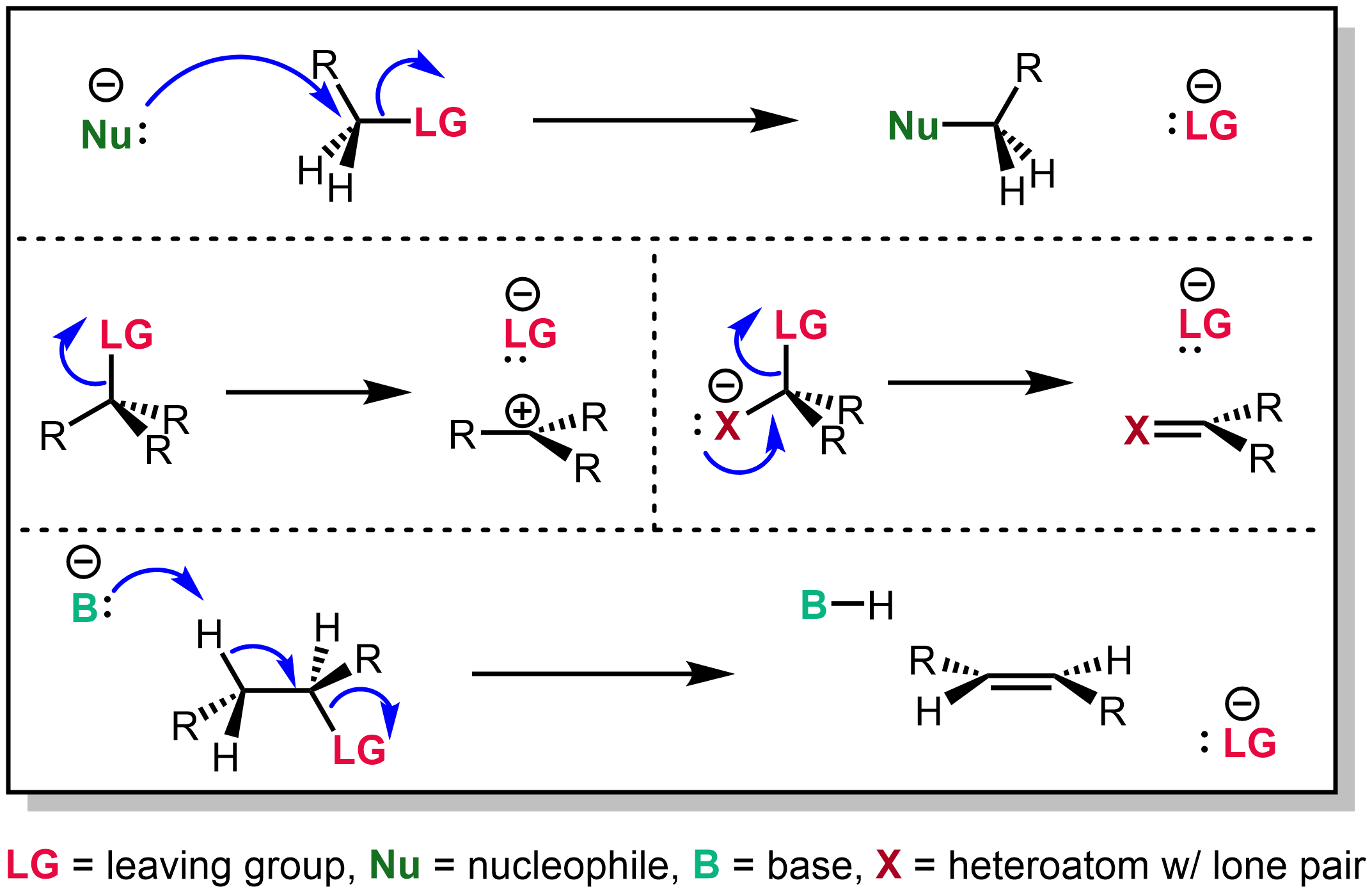
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and '' electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''catio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN1 Reaction
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction occurs. In inorganic chemistry, the SN1 reaction is often known as the ''dissociative substitution''. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schiff Base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. where R' ≠ H). A number of special naming systems exist for these compounds. For instance a Schiff base derived from an aniline, where is a phenyl or a substituted phenyl, can be called an ''anil'', while bis-compounds are often referred to as salen-type compounds. The term Schiff base is normally applied to these compounds when they are being used as ligands to form coordination complexes with metal ions. Such complexes occur naturally, for instance in corrin, but the majority of Schiff bases are artificial and are used to form many important catalysts, such as Jacobsen's catalyst. Synthesis Schiff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |