|
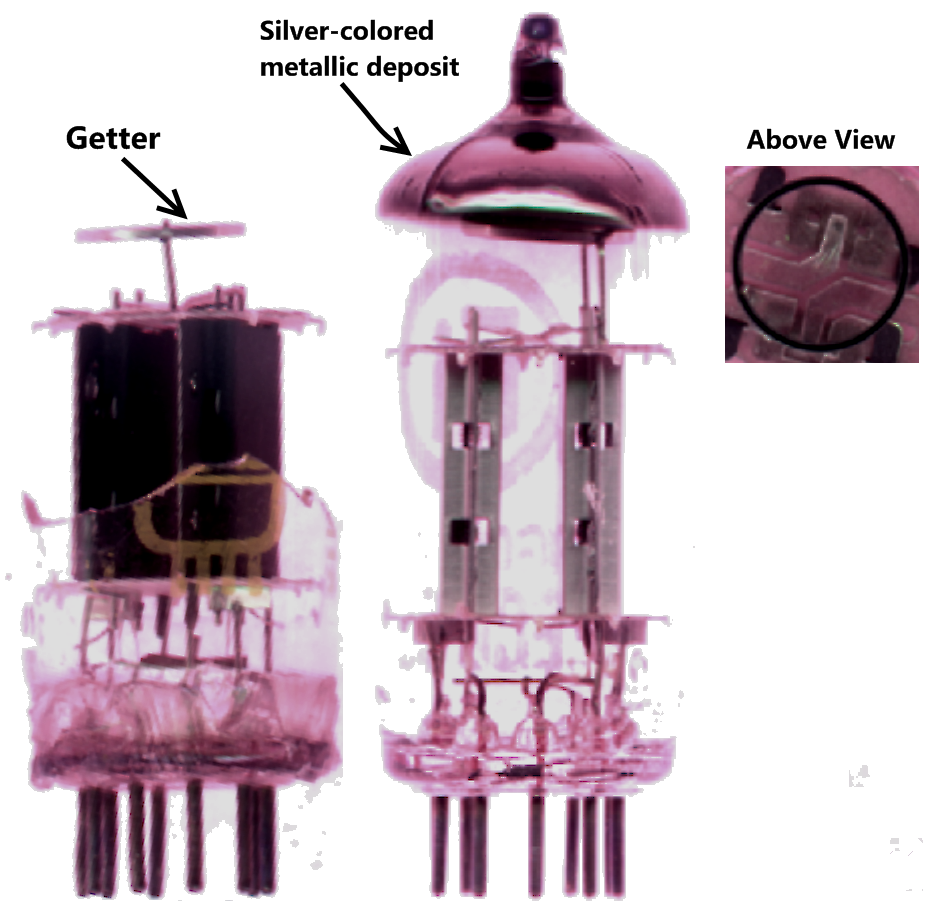
Getter
A getter is a deposit of reactive material that is placed inside a vacuum system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by . Thus the getter removes small amounts of gas from the evacuated space. The getter is usually a coating applied to a surface within the evacuated chamber. A vacuum is initially created by connecting a container to a vacuum pump. After achieving a sufficient vacuum, the container can be sealed, or the vacuum pump can be left running. Getters are especially important in sealed systems, such as vacuum tubes, including cathode ray tubes (CRTs), Vacuum Insulating Glass (or Vacuum Glass) and vacuum insulated panels, which must maintain a vacuum for a long time. This is because the inner surfaces of the container release adsorbed gases for a long time after the vacuum is established. The getter continually removes residues of a reactive gas, such as oxygen, as long as it is desorbed f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Getter Diagram
A getter is a deposit of reactive material that is placed inside a vacuum system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by . Thus the getter removes small amounts of gas from the evacuated space. The getter is usually a coating applied to a surface within the evacuated chamber. A vacuum is initially created by connecting a container to a vacuum pump. After achieving a sufficient vacuum, the container can be sealed, or the vacuum pump can be left running. Getters are especially important in sealed systems, such as vacuum tubes, including cathode ray tubes (CRTs), Vacuum Insulating Glass (or Vacuum Glass) and vacuum insulated panels, which must maintain a vacuum for a long time. This is because the inner surfaces of the container release adsorbed gases for a long time after the vacuum is established. The getter continually removes residues of a reactive gas, such as oxygen, as long as it is desorbed f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplifier, amplification and current rectifier, rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tubes
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplification and current rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—from the cathode to the anode. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode Ray Tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), pictures (television set, computer monitor), radar targets, or other phenomena. A CRT on a television set is commonly called a picture tube. CRTs have also been used as memory devices, in which case the screen is not intended to be visible to an observer. The term ''cathode ray'' was used to describe electron beams when they were first discovered, before it was understood that what was emitted from the cathode was a beam of electrons. In CRT television sets and computer monitors, the entire front area of the tube is scanned repeatedly and systematically in a fixed pattern called a raster. In color devices, an image is produced by controlling the intensity of each of three electron beams, one for each additive primary color (red, green, and bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Pump
A vacuum pump is a device that draws gas molecules from a sealed volume in order to leave behind a partial vacuum. The job of a vacuum pump is to generate a relative vacuum within a capacity. The first vacuum pump was invented in 1650 by Otto von Guericke, and was preceded by the suction pump, which dates to antiquity. History Early pumps The predecessor to the vacuum pump was the suction pump. Dual-action suction pumps were found in the city of Pompeii. Arabic engineer Al-Jazari later described dual-action suction pumps as part of water-raising machines in the 13th century. He also said that a suction pump was used in siphons to discharge Greek fire. The suction pump later appeared in medieval Europe from the 15th century.Donald Routledge Hill (1996), ''A History of Engineering in Classical and Medieval Times'', Routledge, pp. 143 & 150-2Donald Routledge Hill, "Mechanical Engineering in the Medieval Near East", ''Scientific American'', May 1991, pp. 64-69 (cf. Donald Ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are baryte ( barium sulfate, BaSO4) and witherite (barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1774, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Sublimation Pump
A titanium sublimation pump (TSP) is a type of vacuum pump used to remove residual gas in ultra-high vacuum systems, maintaining the vacuum. Principle of operation Its construction and principle of operation is simple. It consists of a titanium filament through which a high current (typically around 40 A) is passed periodically. This current causes the filament to reach the sublimation temperature of titanium, and hence the surrounding chamber walls become coated with a thin film of clean titanium. Since clean titanium is very reactive, components of the residual gas in the chamber which collide with the chamber wall are likely to react and to form a stable, solid product. Thus the gas pressure in the chamber is reduced.VG Scienta retrieved 8 April 2009 After ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Azide
Barium azide is an inorganic azide with the formula . It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide. Preparation Barium azide may be prepared by reacting sodium azide with a soluble barium salt. Care should be taken to prevent large crystals from forming in the solution as barium azide crystals will explode if subjected to friction/shock or if fully dried. The product should be stored submerged in ethanol. Uses Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates. : It can also be used as a source for high pure nitrogen by heating: : This reaction liberates metallic barium, which is used as a getter A getter is a deposit of reactive material that is placed inside a vacuum system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by . Thus the g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often discuss ideal test results that would occur in a ''perfect'' vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure. The Latin term ''in vacuo'' is used to describe an object that is surrounded by a vacuum. The ''quality'' of a partial vacuum refers to how closely it approaches a perfect vacuum. Other things equal, lower gas pressure means higher-quality vacuum. For example, a typical vacuum cleaner produces enough suction to reduce air pressure by around 20%. But hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |