|
Gas Pressure (factors)
The gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between pressure, volume and temperature of a sample of gas could be obtained which would hold to approximation for all gases. Boyle's law In 1662 Robert Boyle studied the relationship between volume and pressure of a gas of fixed amount at constant temperature. He observed that volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature. Boyle's law, published in 1662, states that, at constant temperature, the product of the pressure and volume of a given mass of an ideal gas in a closed system is always constant. It can be verified experimentally using a pressure gauge and a variable volume container. It can also be derived from the kinetic theory of gases: if a container, with a fixed number of molecules inside, is reduced in volume, more molecules will strike a given area of the sides of the container per unit time, causing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johannes Diderik Van Der Waals
Johannes Diderik van der Waals (; 23 November 1837 – 8 March 1923) was a Dutch theoretical physicist and thermodynamicist famous for his pioneering work on the equation of state for gases and liquids. Van der Waals started his career as a school teacher. He became the first physics professor of the University of Amsterdam when in 1877 the old Athenaeum was upgraded to Municipal University. Van der Waals won the 1910 Nobel Prize in physics for his work on the equation of state for gases and liquids. His name is primarily associated with the Van der Waals equation of state that describes the behavior of gases and their condensation to the liquid phase. His name is also associated with Van der Waals forces (forces between stable molecules), with Van der Waals molecules (small molecular clusters bound by Van der Waals forces), and with Van der Waals radii (sizes of molecules). As James Clerk Maxwell said, "there can be no doubt that the name of Van der Waals will soon be among th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Real Gas Law
Real may refer to: Currencies * Brazilian real (R$) * Central American Republic real * Mexican real * Portuguese real * Spanish real * Spanish colonial real Music Albums * Real (L'Arc-en-Ciel album), ''Real'' (L'Arc-en-Ciel album) (2000) * Real (Bright album), ''Real'' (Bright album) (2010) * Real (Belinda Carlisle album), ''Real'' (Belinda Carlisle album) (1993) * Real (Gorgon City EP), ''Real'' (Gorgon City EP) (2013) * Real (IU EP), ''Real'' (IU EP) (2010) * Real (Ivy Queen album), ''Real'' (Ivy Queen album) (2004) * Real (Mika Nakashima album), ''Real'' (Mika Nakashima album) (2013) * Real (Ednita Nazario album), ''Real'' (Ednita Nazario album) (2007) * Real (Jodie Resther album), ''Real'' (Jodie Resther album), a 2000 album by Jodie Resther * Real (Michael Sweet album), ''Real'' (Michael Sweet album) (1995) * Real (The Word Alive album), ''Real'' (The Word Alive album) (2014) * ''Real'', a 2002 album by Israel Houghton recording as Israel & New Breed Songs * Real (Goo Goo Do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry's Law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century. An example where Henry's law is at play is in the depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that changes during decompression, leading to decompression sickness. An everyday example is given by one's experience with carbonated soft drinks, which contain dissolved carbon dioxide. Before opening, the gas above the drink in its container is almost pure carbon dioxide, at a pressure higher than atmospheric pressure. After the bottle is opened, this gas escapes, moving the partial pressure of carbon dioxide above the liquid to be much lower, resulting in degassing as the dissolved carbon dioxide comes out of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Gas Volume
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amagat's Law
Amagat's law or the Law of Partial Volumes describes the behaviour and properties of mixtures of ideal (as well as some cases of non-ideal) gases. It is of use in chemistry and thermodynamics. It is named after Emile Amagat. Overview Amagat's law states that the extensive volume ''V = Nv'' of a gas mixture is equal to the sum of volumes ''Vi'' of the ''K'' component gases, if the temperature ''T'' and the pressure ''p'' remain the same: : N\, v(T, p) = \sum_^K N_i\, v_i(T, p). This is the experimental expression of volume as an extensive quantity. According to Amagat's law of partial volume, the total volume of a non-reacting mixture of gases at constant temperature and pressure should be equal to the sum of the individual partial volumes of the constituent gases. So if V_1, V_2, \dots, V_n are considered to be the partial volumes of components in the gaseous mixture, then the total volume V would be represented as: :V = V_1 + V_2 + V_3 + \dots + V_n = \sum_ V_i Both Amagat's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressures
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
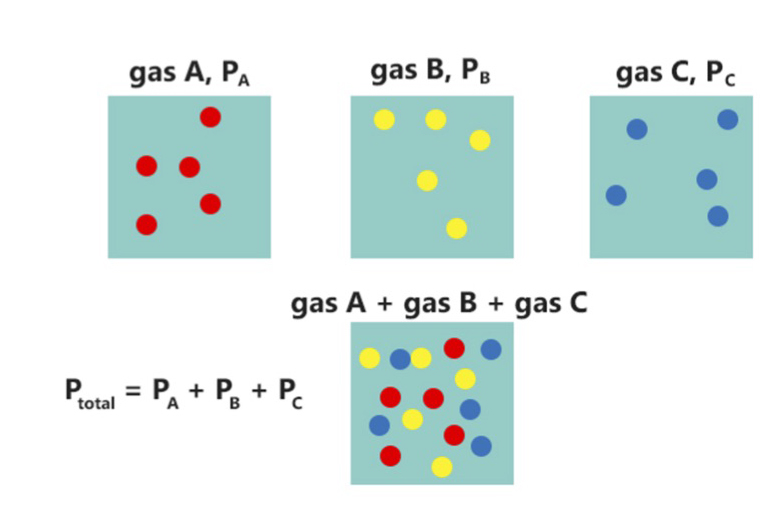
Dalton's Law
Dalton's law (also called Dalton's law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This empirical law was observed by John Dalton in 1801 and published in 1802.J. Dalton (1802)"Essay IV. On the expansion of elastic fluids by heat,"''Memoirs of the Literary and Philosophical Society of Manchester'', vol. 5, pt. 2, pages 595–602; see page 600. Dalton's law is related to the ideal gas laws. Formula Mathematically, the pressure of a mixture of non-reactive gases can be defined as the summation: p_\text = \sum_^n p_i = p_1+p_2+p_3+\cdots+p_n where ''p''1, ''p''2, ..., ''pn'' represent the partial pressures of each component. p_ = p_\text x_i where ''xi'' is the mole fraction of the ''i''th component in the total mixture of ''n'' components . Volume-based concentration The relationship below provides a way to determine the volume-based concentration of any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graham's Law
Graham's law of effusion (also called Graham's law of diffusion) was formulated by Scottish physical chemist Thomas Graham (chemist), Thomas Graham in 1848.Keith J. Laidler and John M. Meiser, ''Physical Chemistry'' (Benjamin/Cummings 1982), pp. 18–19 Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the molar mass of its particles. This formula is stated as: :=\sqrt, where: :Rate1 is the rate of effusion for the first gas. (volume or number of moles per unit time). :Rate2 is the rate of effusion for the second gas. :''M1'' is the molar mass of gas 1 :''M2'' is the molar mass of gas 2. Graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. Thus, if the molecular weight of one gas is four times that of another, it would diffuse through a porous plug or escape through a small pinhole in a vessel at half the rate of the other (heavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |