|
Green Cross (chemical Warfare)
Green Cross (Grünkreuz) is a World War I chemical warfare pulmonary agent consisting of chloropicrin (PS, Aquinite, Klop), phosgene (CG, Collongite) and/or trichloromethyl chloroformate (Surpalite, Perstoff). Green Cross is also a generic World War I German marking for artillery shells with pulmonary agents (chemical payload affecting the lungs). The tip of the projectile with the fuse end painted green and a green cross at the bottom of the cartridge. Other Green Cross mixtures were based on phosgene and/or diphosgene. The first use of Green Cross was on May 31 1915 in a German offensive in Ypres. The mixture was chlorine-phosgene, with 95% and 5%. See also * Blue Cross (chemical warfare) * Yellow Cross (chemical warfare) * White Cross (chemical warfare) White Cross (Weiẞkreuz) is a World War I chemical warfare agent consisting of one or more lachrymatory agents: bromoacetone (BA), bromobenzyl cyanide (Camite), bromomethyl ethyl ketone (homomartonite, Bn-stoff), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fighting occurring throughout Europe, the Middle East, Africa, the Pacific, and parts of Asia. An estimated 9 million soldiers were killed in combat, plus another 23 million wounded, while 5 million civilians died as a result of military action, hunger, and disease. Millions more died in genocides within the Ottoman Empire and in the 1918 influenza pandemic, which was exacerbated by the movement of combatants during the war. Prior to 1914, the European great powers were divided between the Triple Entente (comprising France, Russia, and Britain) and the Triple Alliance (containing Germany, Austria-Hungary, and Italy). Tensions in the Balkans came to a head on 28 June 1914, following the assassination of Archduke Franz Ferdin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Warfare
Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare, biological warfare and radiological warfare, which together make up CBRN, the military acronym for chemical, biological, radiological, and nuclear (warfare or weapons), all of which are considered "weapons of mass destruction" (WMDs), a term that contrasts with conventional weapons. The use of chemical weapons is prohibited under customary international humanitarian law. Definition Chemical warfare is different from the use of conventional weapons or nuclear weapons because the destructive effects of chemical weapons are not primarily due to any explosive force. The offensive use of living organisms (such as anthrax) is considered biological warfare rather than chemical warfare; however, the use of nonliving toxic products produced by living organisms (e.g. toxins such as botulinum toxin, ricin, and saxitoxin) ''is'' consider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulmonary Agent
A pulmonary agent, or choking agent, is a chemical weapon agent designed to impede a victim's ability to breathe. They operate by causing a build-up of fluids in the lungs, which then leads to suffocation. Exposure to the eyes and skin tends to be corrosive, causing blurred vision and severe deep burns. Inhalation of these agents cause burning of the throat, coughing, vomiting, headache, pain in chest, tightness in chest, and respiratory and circulatory failure. Examples of pulmonary agents include: *Chlorine gas *Chloropicrin (PS) *Diphosgene (DP) * Phosgene (CG) *Disulfur decafluoride *Perfluoroisobutene * Acrolein *Diphenylcyanoarsine Phosgene is the most dangerous commonly used pulmonary agent (although disulfur decafluoride and perfluoroisobutene are both even more dangerous, with respectively 4 and 10 times the lethality of phosgene, neither is widely used). It is a colorless gas under ordinary conditions. It has a vapor density 3.4 times greater than that of air, allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloropicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2. Synthesis Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid: : HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar. Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite: : H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH or by the reaction of chloroform with nitric acid: : CHCl3 + HNO3 → CCl3NO2 + H2O Properties Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole. Pure chloropicrin is a colorless liquid, with a boiling po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics. Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It was a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas. It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform. Structure and basic properties Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl angle is 111 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloromethyl Chloroformate
Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the organic synthesis, synthesis of organic Chemical compound, compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas. Production and uses Diphosgene is prepared by Radical (chemistry), radical chlorination of methyl chloroformate under ultraviolet, UV light: :Cl-CO-OCH3 + 3 Cl2 —(hv)→ Cl-CO-OCCl3 + 3 HCl Another method is the radical chlorination of methyl formate: :H-CO-OCH3 + 4 Cl2 —(hv)→ Cl-CO-OCCl3 + 4 HCl Diphosgene converts to phosgene upon heating or upon catalysis with charcoal. It is thus useful for reactions traditionally relying on phosgene. For example, it convert amines into isocyanates, secondary amines into carbamoyl chlorides, carboxylic acids into acid chlorides, and formamides into isocyanides. Diphosgene serves as a source of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
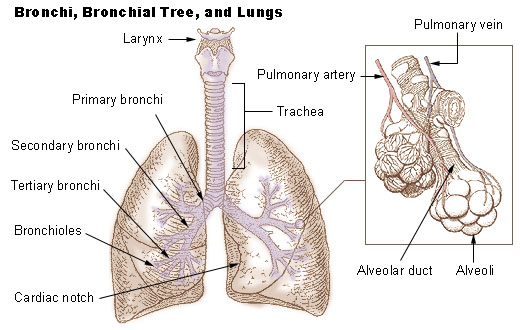
Lungs
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their different muscles to support and foster breathing. In earlier tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the main muscle of respiration that drives breathing is the diaphragm. The lungs also provide airflow that makes vocal sounds including human speech possible. Humans have two lungs, one on the left and one on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuse (explosive)
In an explosive, pyrotechnic device, or military munition, a fuse (or fuze) is the part of the device that initiates function. In common usage, the word fuse is used indiscriminately. However, when being specific (and in particular in a military context), the term ''fuse'' describes a simple pyrotechnic initiating device, like the cord on a firecracker whereas the term ''fuze'' is used when referring to a more sophisticated ignition device incorporating mechanical and/or electronic components, such as a proximity fuze for an M107 artillery shell, magnetic or acoustic fuze on a sea mine, spring-loaded grenade fuze, pencil detonator, or anti-handling device. History Documented evidence suggests that the earliest fuses were first used by the Song Chinese between the 10th and 12th centuries. After the Chinese invented gunpowder, they began adapting its explosive properties for use in military technology. By 1044 they were using gunpowder in simple grenades, bombs, and flamethro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosgene
Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas. Production and uses Diphosgene is prepared by radical chlorination of methyl chloroformate under UV light: :Cl-CO-OCH3 + 3 Cl2 —(hv)→ Cl-CO-OCCl3 + 3 HCl Another method is the radical chlorination of methyl formate: :H-CO-OCH3 + 4 Cl2 —(hv)→ Cl-CO-OCCl3 + 4 HCl Diphosgene converts to phosgene upon heating or upon catalysis with charcoal. It is thus useful for reactions traditionally relying on phosgene. For example, it convert amines into isocyanates, secondary amines into carbamoyl chlorides, carboxylic acids into acid chlorides, and formamides into isocyanides. Diphosgene serves as a source of two equivalents of phosgene: :2 RNH2 + ClCO2CCl3 → 2 RNCO + 4 HCl W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
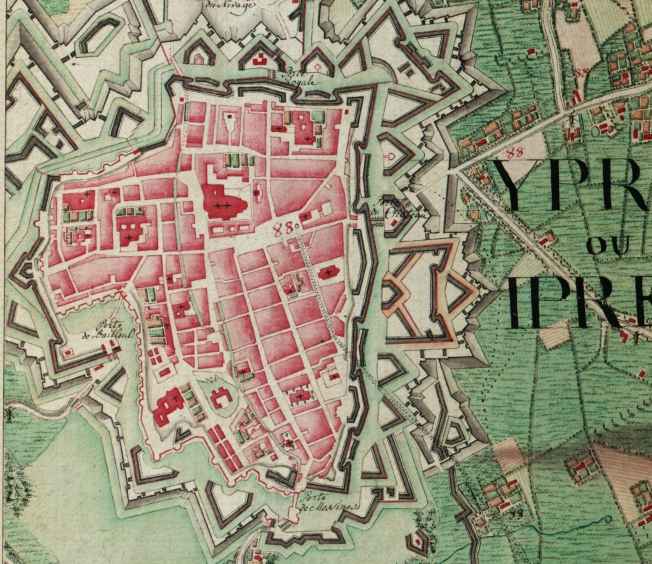
Ypres
Ypres ( , ; nl, Ieper ; vls, Yper; german: Ypern ) is a Belgian city and municipality in the province of West Flanders. Though the Dutch name is the official one, the city's French name is most commonly used in English. The municipality comprises the city of Ypres/Ieper and the villages of Boezinge, Brielen, Dikkebus, Elverdinge, Hollebeke, Sint-Jan, Vlamertinge, Voormezele, Zillebeke, and Zuidschote. Together, they are home to about 34,900 inhabitants. During the First World War, Ypres (or "Wipers" as it was commonly known by the British troops) was the centre of the Battles of Ypres between German and Allied forces. History Origins before First World War Ypres is an ancient town, known to have been raided by the Romans in the first century BC. It is first mentioned by name in 1066 and is probably named after the river Ieperlee on the banks of which it was founded. During the Middle Ages, Ypres was a prosperous Flemish city with a population of 40,000 in 1200 AD, renow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blue Cross (chemical Warfare)
Blue Cross (Blaukreuz) is a World War I chemical warfare agent consisting of diphenylchloroarsine (DA, Clark I), diphenylcyanoarsine (CDA, Clark II), ethyldichloroarsine (Dick), and/or methyldichloroarsine (Methyldick). Clark I and Clark II were the main agents used. Clark I was used with Green Cross munition earlier; however for the first time it was used as a standalone agent in the night from July 10 to July 11 1917 at Nieuwpoort, Belgium, during "Operation Strandfest". The artillery munition used as a delivery vehicle contained a large amount of glass spheres closed with a cork and sealed with trinitrotoluene (TNT). Later N-ethylcarbazole was added. Depending on the caliber, the munition contained between 7 and 120 kilograms of the agent. Blue Cross is also a generic World War I German marking for artillery shells with chemical payload affecting the upper respiratory tract. See also * Green Cross (chemical warfare) * Yellow Cross (chemical warfare) * White Cross (chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_-_Gassed_-_Google_Art_Project.jpg)