|
Goldschmidt Tolerance Factor
Goldschmidt's tolerance factor (from the German word ''Toleranzfaktor'') is an indicator for the stability and distortion of crystal structures. It was originally only used to describe the perovskite ABO3 structure, but now tolerance factors are also used for ilmenite. Alternatively the tolerance factor can be used to calculate the compatibility of an ion with a crystal structure. The first description of the tolerance factor for perovskite was made by Victor Moritz Goldschmidt in 1926. Mathematical expression The Goldschmidt tolerance factor (t) is a dimensionless number that is calculated from the ratio of the ionic radii: In an ideal cubic perovskite structure, the lattice parameter (i.e., length) of the unit cell (a) can be calculated using the following equation: Perovskite structure The perovskite structure has the following tolerance factors (t): See also * Goldschmidt classification * Victor Goldschmidt Victor Moritz Goldschmidt (27 January 1888 in ZĂ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
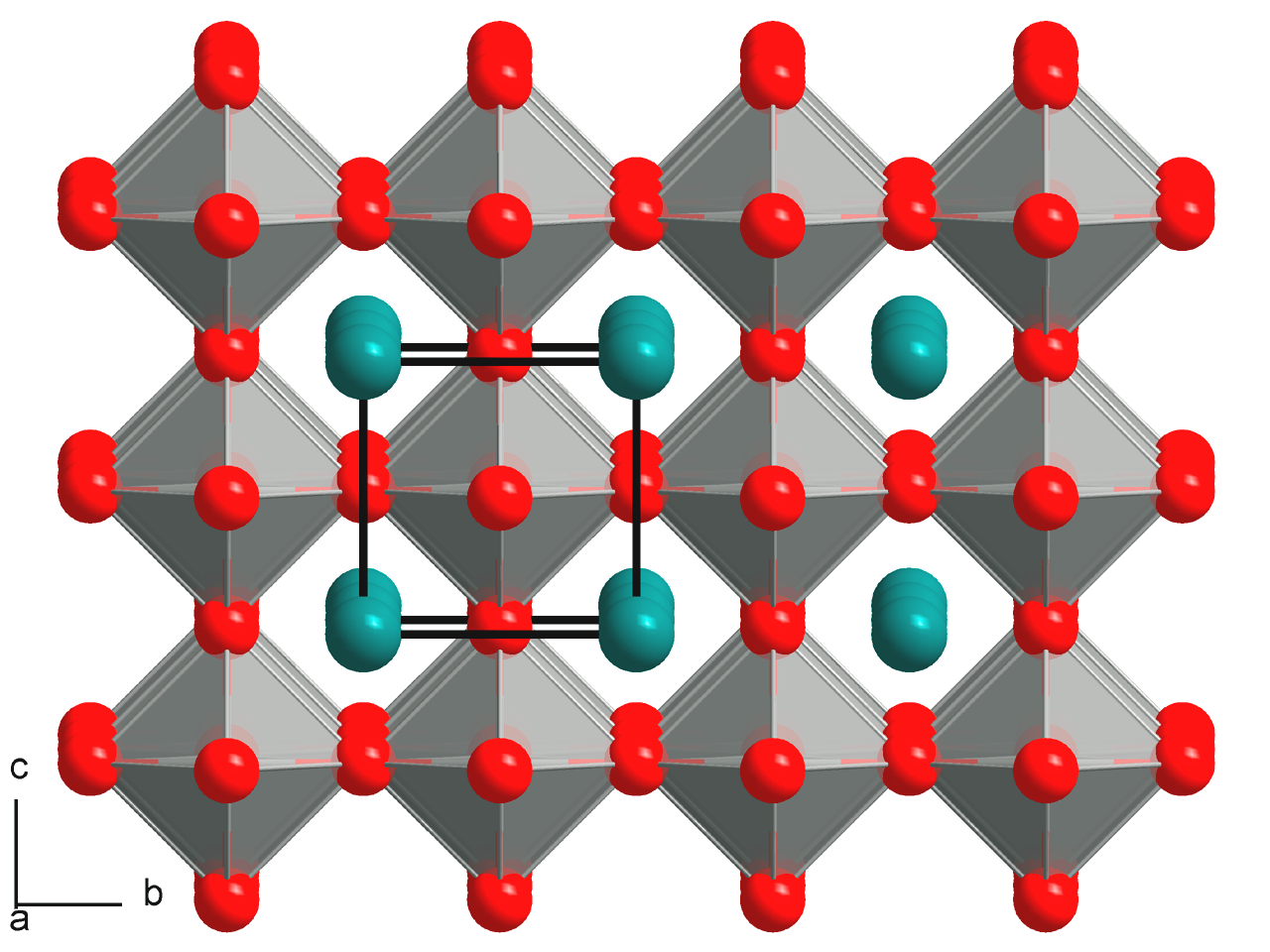
Perovskite (structure)
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Perovskite Structure
Cubic may refer to: Science and mathematics * Cube (algebra), "cubic" measurement * Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex ** Cubic crystal system, a crystal system where the unit cell is in the shape of a cube * Cubic function, a polynomial function of degree three * Cubic equation, a polynomial equation (reducible to ''ax''3 + ''bx''2 + ''cx'' + ''d'' = 0) * Cubic form, a homogeneous polynomial of degree 3 * Cubic graph (mathematics - graph theory), a graph where all vertices have degree 3 * Cubic plane curve (mathematics), a plane algebraic curve ''C'' defined by a cubic equation * Cubic reciprocity (mathematics - number theory), a theorem analogous to quadratic reciprocity * Cubic surface, an algebraic surface in three-dimensional space * Cubic zirconia, in geology, a mineral that is widely synthesized for use as a diamond simulacra * CUBIC, a histology method Computing * Cubic IDE, a modular deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Goldschmidt Classification
The Goldschmidt classification, developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile (sulfide ore-loving or chalcogen-loving), and atmophile (gas-loving) or volatile (the element, or a compound in which it occurs, is liquid or gaseous at ambient surface conditions). Some elements have affinities to more than one phase. The main affinity is given in the table below and a discussion of each group follows that table. Lithophile elements Lithophile elements are those that remain on or close to the surface because they combine readily with oxygen, forming compounds that do not sink into the Earth's core. The lithophile elements include: Al, B, Ba, Be, Br, Ca, Cl, Cr, Cs, F, I, Hf, K, Li, Mg, Na, Nb, O, P, Rb, Sc, Si, Sr, Ta, Th, Ti, U, V, Y, Zr, W and the lanthanide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ilmenite
Ilmenite is a titanium-iron oxide mineral with the idealized formula . It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics. Structure and properties Ilmenite is a heavy (specific gravity 4.7), moderately hard (Mohs hardness 5.6 to 6), opaque black mineral with a submetallic luster. It is almost always massive, with thick tabular crystals being quite rare. It shows no discernible cleavage, breaking instead with a conchoidal to uneven fracture. Ilmenite crystallizes in the trigonal system with space group ''R''. The ilmenite crystal structure consists of an ordered derivative of the corundum structure; in corundum all cations are identical but in ilmenite Fe2+ and Ti4+ ions occupy alternating layers perpendicular to the trigonal c axis. Pure ilmenite is paramagnetic (showing only very weak attrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CaTiO3 Perovskite Structure
Calcium titanate is an inorganic compound with the chemical formula Ca Ti O3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities. Synthesis CaTiO3 can be prepared by the combination of CaO and TiO2 at temperatures >1300 °C. Sol-gel processes has been used to make a more pure substance, as well as lowering the synthesis temperature. These compounds synthesized are more compressible due to the powders from the sol-gel process as well and bring it closer to its calculated density (~4.04 g/ml). Structure Calcium titanate is obtained as orthorhombic crystals, more specifically perovskite structure. In this motif, the Ti(IV) centers are octahedral and the Ca2+ centers occupy a cage of 12 oxygen centres. Many useful materials adopt related structures, e.g. barium titanate or variations of the structure, e.g. yttrium barium copp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CaTiO3
Calcium titanate is an inorganic compound with the chemical formula Ca Ti O3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities. Synthesis CaTiO3 can be prepared by the combination of CaO and TiO2 at temperatures >1300 °C. Sol-gel processes has been used to make a more pure substance, as well as lowering the synthesis temperature. These compounds synthesized are more compressible due to the powders from the sol-gel process as well and bring it closer to its calculated density (~4.04 g/ml). Structure Calcium titanate is obtained as orthorhombic crystals, more specifically perovskite structure. In this motif, the Ti(IV) centers are octahedral and the Ca2+ centers occupy a cage of 12 oxygen centres. Many useful materials adopt related structures, e.g. barium titanate or variations of the structure, e.g. yttrium barium copp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhombohedral Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SrTiO3
Strontium titanate is an oxide of strontium and titanium with the chemical formula Sr Ti O3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics. The name ''tausonite'' was given in honour of Lev Vladimirovich Tauson (1917–1989), a Russian geochemist. Disus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ilmenite
Ilmenite is a titanium-iron oxide mineral with the idealized formula . It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics. Structure and properties Ilmenite is a heavy (specific gravity 4.7), moderately hard (Mohs hardness 5.6 to 6), opaque black mineral with a submetallic luster. It is almost always massive, with thick tabular crystals being quite rare. It shows no discernible cleavage, breaking instead with a conchoidal to uneven fracture. Ilmenite crystallizes in the trigonal system with space group ''R''. The ilmenite crystal structure consists of an ordered derivative of the corundum structure; in corundum all cations are identical but in ilmenite Fe2+ and Ti4+ ions occupy alternating layers perpendicular to the trigonal c axis. Pure ilmenite is paramagnetic (showing only very weak attrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BaTiO3
Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics. Structure The solid exists in one of four polymorphs depending on temperature. From high to low temperature, these crystal symmetries of the four polymorphs are cubic, tetragonal, orthorhombic and rhombohedral crystal structure. All of these phases exhibit the ferroelectric effect apart from the cubic phase. The high temperature cubic phase is easiest to describe, as it consists of regular corner-sharing octahedral TiO6 units that define a cube with O vertices and Ti-O-Ti edges. In the cubic phase, Ba2+ is located at the center of the cube, with a nominal coordination number of 12. Lower symmetry phases are stabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |