|
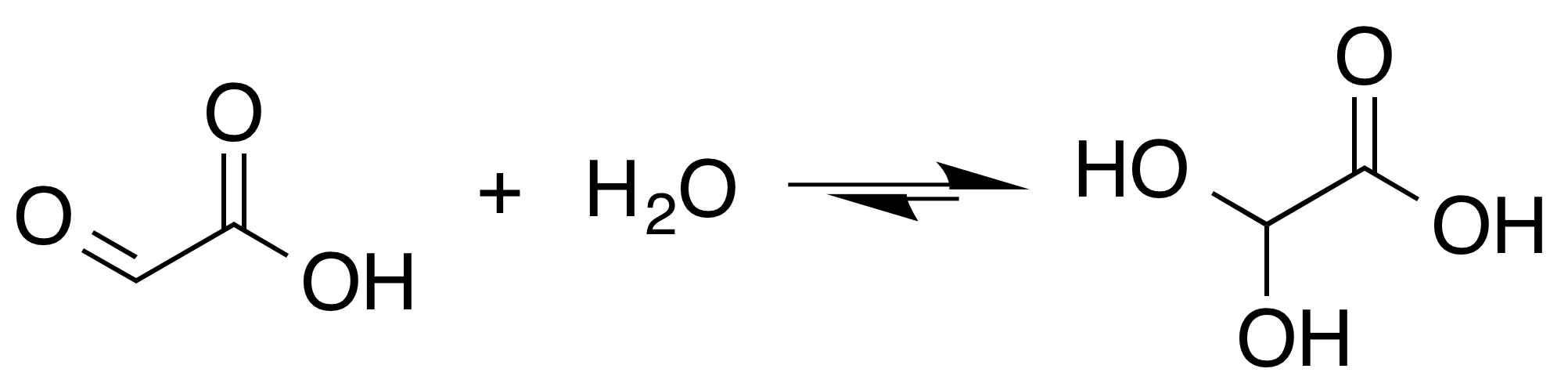
Glyoxal
Glyoxal is an organic compound In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ... with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.O'Neil, M.J. (2001): ''The Merck Index'', 13th Edition, page 803. Pure glyoxal is not commonly encountered because glyoxal is usually handled as a 40% aqueous solution (density near 1.24 g/mL). It forms a series of hydrates, including oligomers. For many purposes, these hydrated oligomers behave equivalently to glyoxal. Glyoxal is produced industrially as a precursor to many products. Production Glyoxal was first prepared and named by the German-British ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylglyoxal
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase. Chemical structure Gaseous methylglyoxal has two carbonyl groups, an aldehyde and a ketone. In the presence of water, it exists as hydrates and oligomers. The formation of these hydrates is indicative of the high reactivity of MGO, which is relevant to its biological behavior. Biochemistry Biosynthesis and biodegradation In organisms, methylglyoxal is formed as a side-product of several metabolic pathways. Methylglyoxal mainly arises as side products of glycolysis involving glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. It is also thought to arise via the degradation of acetone and threonine. Illustrative of the myriad pathways to MGO, aristolochic acid caused 12-f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heinrich Debus (chemist)
Heinrich Debus (13 July 1824 – 9 December 1915) was a German chemist. Education and career In 1838, he attended a trade school in Kassel, where he was taught by Robert Wilhelm Bunsen. He studied chemistry from 1845 to 1848 in Marburg, and served as Bunsen's assistant from 1847. In 1848, he earned his doctorate by investigating a red madder dye. He completed his habilitation in 1851 after Bunsen left for Breslau. At the suggestion of Frederick Augustus Genth, Debus was named Bunsen's successor at Marburg. Later in 1851 he left for England to be a chemistry teacher at Queenwood College followed from 1868 to 1870 by the position as Science Master at Clifton College, Bristol. He then taught at Guy's Hospital, London from 1870 until appointed foundation Professor of Chemistry in 1873 at the new Royal Naval College, Greenwich, where he remained until his retirement and return to Germany. In 1858, Debus first synthesized imidazole from glyoxal, ammonia, and formaldehyde. The Debus syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialdehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Aldehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenious Acid
Selenous acid (or selenious acid) is the chemical compound with the formula . Structurally, it is more accurately described by . It is the principal oxoacid of selenium; the other being selenic acid. Formation and properties Selenous acid is analogous to sulfurous acid, but it is more readily isolated. Selenous acid is easily formed upon the addition of selenium dioxide to water. As a crystalline solid, the compound can be seen as pyramidal molecules that are interconnected with hydrogen bonds. In solution it is a diprotic acid: : (p''K''a = 2.62) : (p''K''a = 8.32) It is moderately oxidizing in nature, but kinetically slow. In 1 M : : (''E''o = +0.74 V) In 1 M : : (''E''o = −0.37 V) Selenous acid is hygroscopic. Uses The major use is in protecting and changing the color of steel, especially steel parts on firearms. The so-called cold-bluing process uses selenous acid, copper(II) nitrate, and nitric acid to change the color of the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolaldehyde
Glycolaldehyde is the organic compound with the formula . It is the smallest possible molecule that contains both an aldehyde group () and a hydroxyl group (). It is a highly reactive molecule that occurs both in the biosphere and in the interstellar medium. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrates, , it is not generally considered to be a saccharide. Structure Glycolaldehyde as a gas is a simple monomeric structure. As a solid and molten liquid, it exists as a dimer. Collins and George reported the equilibrium of glycolaldehyde in water by using NMR. In aqueous solution, it exists as a mixture of at least four species, which rapidly interconvert. In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide. While not a true sugar, it is the simplest sugar-relate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germany
Germany,, officially the Federal Republic of Germany, is a country in Central Europe. It is the second most populous country in Europe after Russia, and the most populous member state of the European Union. Germany is situated between the Baltic and North seas to the north, and the Alps to the south; it covers an area of , with a population of almost 84 million within its 16 constituent states. Germany borders Denmark to the north, Poland and the Czech Republic to the east, Austria and Switzerland to the south, and France, Luxembourg, Belgium, and the Netherlands to the west. The nation's capital and most populous city is Berlin and its financial centre is Frankfurt; the largest urban area is the Ruhr. Various Germanic tribes have inhabited the northern parts of modern Germany since classical antiquity. A region named Germania was documented before AD 100. In 962, the Kingdom of Germany formed the bulk of the Holy Roman Empire. During the 16th ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
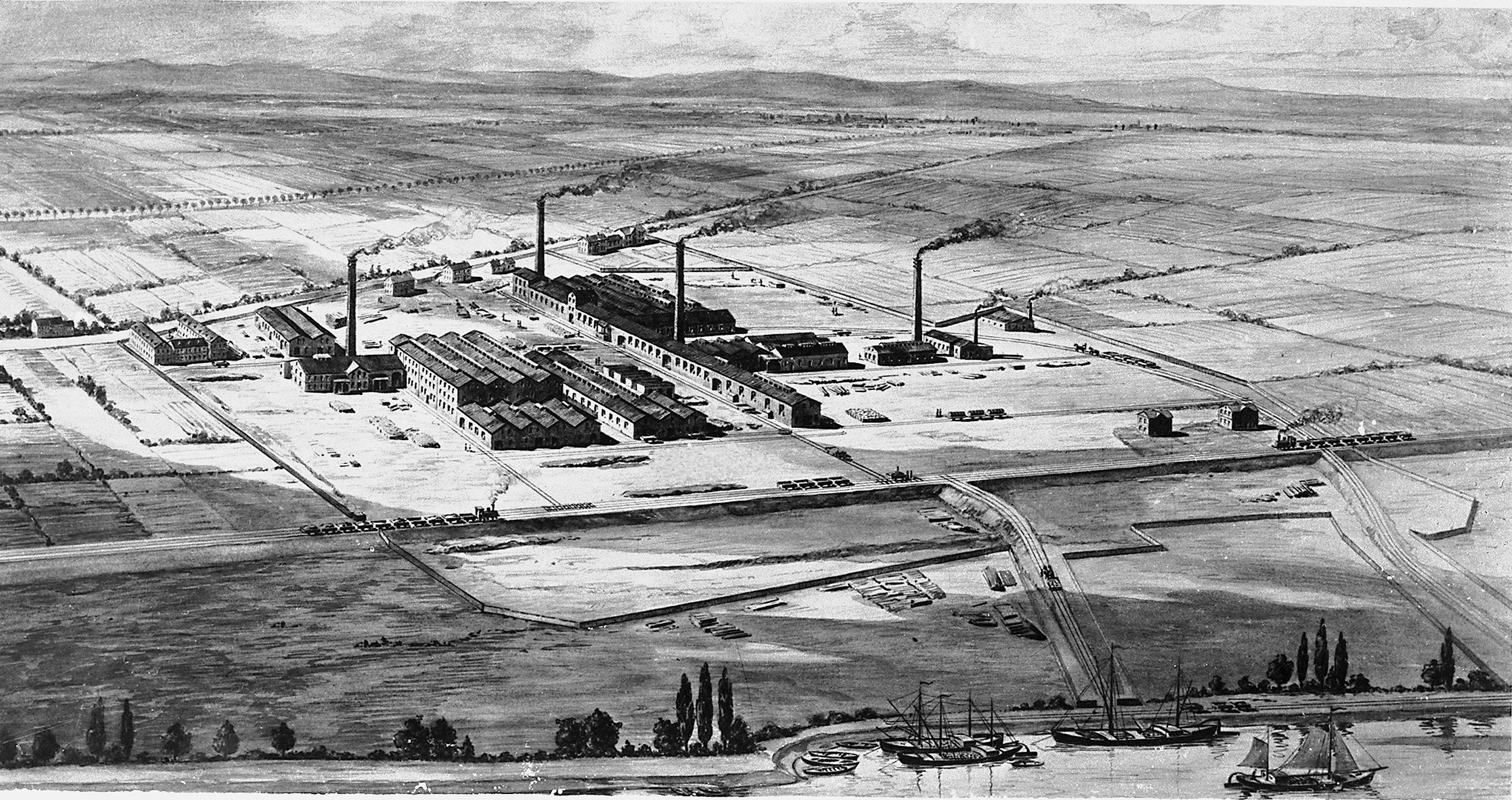
Ludwigshafen
Ludwigshafen, officially Ludwigshafen am Rhein (; meaning " Ludwig's Port upon Rhine"), is a city in the German state of Rhineland-Palatinate, on the river Rhine, opposite Mannheim. With Mannheim, Heidelberg, and the surrounding region, it forms the Rhine Neckar Area. Known primarily as an industrial city, Ludwigshafen is home to BASF, the world's largest chemical producer, and other companies. Among its cultural facilities are the Staatsphilharmonie Rheinland-Pfalz. It is the birthplace and deathplace of the former German chancellor Helmut Kohl. In 2012, Ludwigshafen was classified as a global city with ' Sufficiency' status by the Globalization and World Cities Research Network (GaWC). History Early history In antiquity, Celtic and Germanic tribes settled in the Rhine Neckar area. During the 1st century B.C. the Romans conquered the region, and a Roman auxiliary fort was constructed near the present suburb of Rheingönheim. The Middle Ages saw the foundation of some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF Societas Europaea, SE () is a German multinational corporation, multinational chemical company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany. The BASF Group comprises subsidiary, subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. At the end of 2019, the company employed 117,628 people, with over 54,000 in Germany. , BASF posted sales of €59.3 billion and income from operations before special items of about €4.5 billion. Between 1990 and 2005, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trosly-Breuil
Trosly-Breuil () is a commune in the Oise department in northern France. In 1964, Canadian Jean Vanier invited two men, Raphael Simi and Philippe Seux, to leave the institutions where they lived and live with him in Trosly-Breuil. Their time together led to the establishment of L'Arche at Trosly-Breuil, a community for people with disabilities to live with those who cared for them. Since that time L'Arche communities have been established in fifty countries around the world. See also * Communes of the Oise department The following is a list of the 679 communes of the Oise department of France. The communes cooperate in the following intercommunalities (as of 2020):Communes of Oise {{Oise-geo-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |