|
Glycerate Dehydrogenase
In enzymology, a glycerate dehydrogenase () is an enzyme that catalyzes the chemical reaction :(D)-glycerate + NAD+ \rightleftharpoons hydroxypyruvate + NADH + H+ Thus, the two substrates of this enzyme are (R)-glycerate and NAD+, whereas its 3 products are hydroxypyruvate, NADH, and H+. However, in nature these enzymes have the ability to catalyze the reverse reaction as well. That is, hydroxypyruvate, NADH, and H+ can act as the substrates while (R)-glycerate and NAD+ are formed as products. Additionally, NADPH can take the place of NADH in this reaction. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is (R)-glycerate:NAD+ oxidoreductase. Other names in common use include D-glycerate dehydrogenase, and hydroxypyruvate reductase (due to the reversibility of the reaction). This enzyme participates in glycine, serine and threonine metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymology
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two force ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactate Dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. LDH exists in four distinct enzyme classes. This article is specifically about the NAD(P)-dependent L-lactate dehydrogenase. Other LDHs act on D-lactate and/or are dependent on cytochrome c: D-lactate dehydrogenase (cytochrome) and L-lactate dehydrogenase (cytochrome). LDH is expressed extensively in body tissues, such as blood cells and heart muscle. Because it is released during tissue damage, it is a marker of common injuries and disease such as heart failure. Reaction Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+. It converts pyruvate, the final product of glycolysis, to lactate when oxygen is absent or in short supp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus ''Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Hyperoxaluria
Primary hyperoxaluria is a rare condition (autosomal recessive), resulting in increased excretion of oxalate (up to 600 mg a day from normal 50 mg a day), with oxalate stones being common. Signs and symptoms Primary hyperoxaluria is an autosomal recessive disease, meaning both copies of the gene contain the mutation. Both parents must have one copy of this mutated gene to pass it on to their child, but they do not typically show signs or symptoms of the disease. A single kidney stone in children or recurrent stones in adults is often the first warning sign of primary hyperoxaluria. Other symptoms range from recurrent urinary tract infections, severe abdominal pain or pain in the side, blood in the urine, to chronic kidney disease and kidney failure. The age of symptom onset, progression and severity can vary greatly from one person to another, even among members of the same family. Some individuals may have mild cases that go undiagnosed well into adulthood; others may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactate Dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. LDH exists in four distinct enzyme classes. This article is specifically about the NAD(P)-dependent L-lactate dehydrogenase. Other LDHs act on D-lactate and/or are dependent on cytochrome c: D-lactate dehydrogenase (cytochrome) and L-lactate dehydrogenase (cytochrome). LDH is expressed extensively in body tissues, such as blood cells and heart muscle. Because it is released during tissue damage, it is a marker of common injuries and disease such as heart failure. Reaction Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+. It converts pyruvate, the final product of glycolysis, to lactate when oxygen is absent or in short supp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions from sub-disciplines and related fields, see Glossary of genetics, Glossary of evolutionary biology, Glossary of ecology, and Glossary of scientific naming, or any of the organism-specific glossaries in :Glossaries of biology. A B C D E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolic Acid
Glycolic acid (or hydroxyacetic acid; chemical formula HOCH2CO2H) is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate (sometimes spelled "glycollate") is a salt or ester of glycolic acid. History The name "glycolic acid" was coined in 1848 by French chemist Auguste Laurent (1807–1853). He proposed that the amino acid glycine—which was then called ''glycocolle''—might be the amine of a hypothetical acid, which he called "glycolic acid" (''acide glycolique''). Glycolic acid was first prepared in 1851 by German chemist Adolph Strecker (1822–1871) and Russian chemist Nikolai Nikolaevich Sokolov (1826–1877). They produced it by treating hippuric acid with nitric acid and nitrogen dioxide to form an ester of benzoic acid and glycolic acid (C6H5C(=O)OCH2COOH), which they called "benzoglycolic acid" (''Benzoglykolsäure''; also benzoyl glycolic acid). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
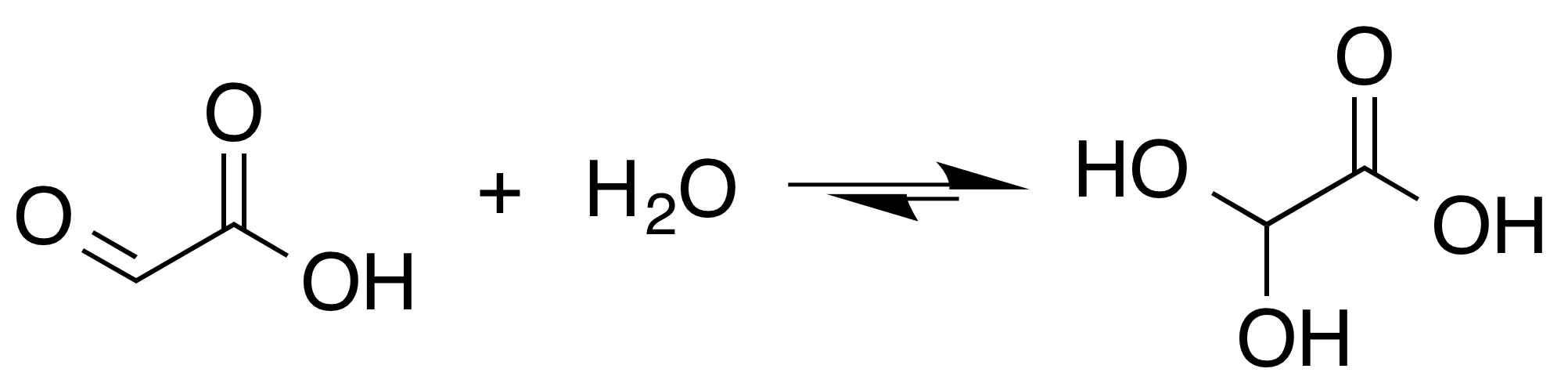
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxylate Reductase
Glyoxylate reductase (), first isolated from spinach leaves, is an enzyme that catalyzes the reduction of glyoxylate to glycolate, using the cofactor NADH or NADPH. The systematic name of this enzyme class is glycolate:NAD+ oxidoreductase. Other names in common use include NADH-glyoxylate reductase, glyoxylic acid reductase, and NADH-dependent glyoxylate reductase. Structure The crystal structure of the glyoxylate reductase enzyme from the hyperthermophilic archeon Pyrococcus horiskoshii OT3 has been reported. The enzyme exists in the dimeric form. Each monomer has two domains: a substrate-binding domain where glyoxylate binds, and a nucleotide-binding domain where the NAD(P)H cofactor binds. Mechanism The enzyme catalyzes the transfer of a hydride from NAD(P)H to glyoxylate, causing a reduction of the substrate to glycolate and an oxidation of the cofactor to NAD(P)+. Figure 2 shows the mechanism for this reaction. It is thought that the two residues Glu270 and Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or intermediates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |