|
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or intermediates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as ''catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate by ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function. Different metabolic pathways function based on the position within a eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the, electron transport chain, and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathways that are character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose below 70 mg/dL (3.9 mmol/L), symptoms associated with hypoglycemia, and resolution of symptoms when blood sugar returns to normal. Hypoglycemia may result in headache, tiredness, clumsiness, trouble talking, confusion, fast heart rate, sweating, shakiness, nervousness, hunger, loss of consciousness, seizures, or death. Symptoms typically come on quickly. The most common cause of hypoglycemia is medications used to treat diabetes such as insulin, sulfonylureas, and biguanides. Risk is greater in diabetics who have eaten less than usual, recently exercised, or consumed alcohol. Other causes of hypoglycemia include severe illness, sepsis, kidney failure, liver disease, hormone deficiency, tumors such as insulinomas or non-B cell tumo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cori Cycle
The Cori cycle (also known as the lactic acid cycle), named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and converted to glucose, which then returns to the muscles and is cyclically metabolized back to lactate. Process Muscular activity requires ATP, which is provided by the breakdown of glycogen in the skeletal muscles. The breakdown of glycogen, known as glycogenolysis, releases glucose in the form of glucose 1-phosphate (G1P). The G1P is converted to G6P by phosphoglucomutase. G6P is readily fed into glycolysis, (or can go into the pentose phosphate pathway if G6P concentration is high) a process that provides ATP to the muscle cells as an energy source. During muscular activity, the store of ATP needs to be constantly replenished. When the supply of oxygen is sufficient, this energy comes from feeding pyruvate, one product of glycolysis, into the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-carbohydrate Diet
Low-carbohydrate diets restrict carbohydrate consumption relative to the average diet. Foods high in carbohydrates (e.g., sugar, bread, pasta) are limited, and replaced with foods containing a higher percentage of fat and protein (e.g., meat, poultry, fish, shellfish, eggs, cheese, nuts, and seeds), as well as low carbohydrate foods (e.g. spinach, kale, chard, collards, and other fibrous vegetables). There is a lack of standardization of how much carbohydrate low-carbohydrate diets must have, and this has complicated research. One definition, from the American Academy of Family Physicians, specifies low-carbohydrate diets as having less than 20% of calories from carbohydrates. There is no good evidence that low-carbohydrate dieting confers any particular health benefits apart from weight loss, where low-carbohydrate diets achieve outcomes similar to other diets, as weight loss is mainly determined by calorie restriction and adherence. An extreme form of low-carbohydrate d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
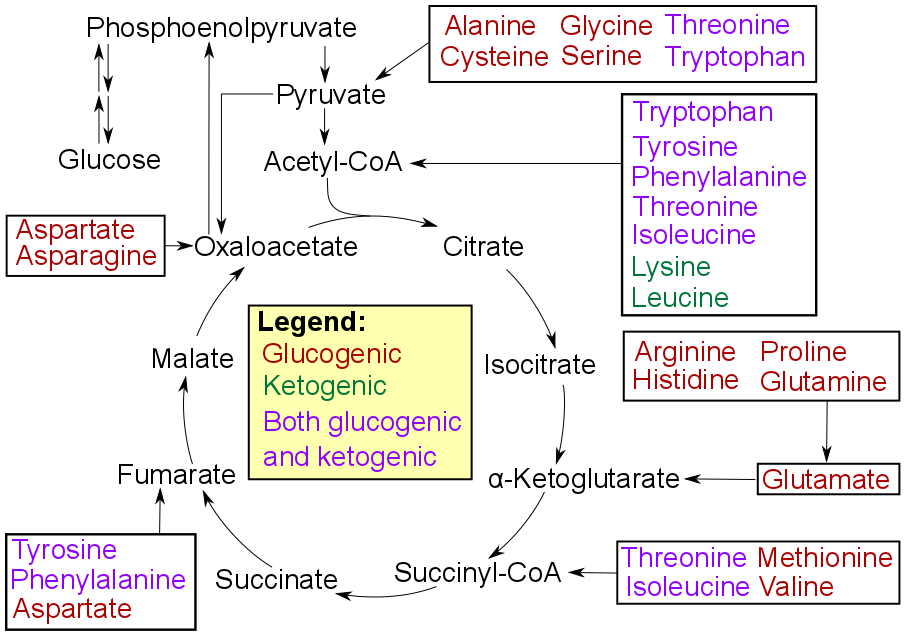
Pyruvic Acid
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organic acid. Jöns Jacob Berzelius characterized this other acid the following year and named pyruvic acid because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may not be different from ''n''), which does not mean the H has covalent bonds with O (for example with , H has a covalent bond with C but not with O). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid). The term is most common in biochemistry, where it is a synonym of saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular wei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase. Mechanism The overall reaction for the breakdown of glycogen to glucose-1-phosphate is: : glycogen(n residues) + Pi glycogen(n-1 residues) + glucose-1-phosphate Here, glycogen phosphorylase cleaves the bond linking a terminal glucose residue to a glycogen branch by substitution of a phosphoryl group for the α →4linkage. Glucose-1-phosphate is converted to glucose-6-phosphate (which often ends up in glycolysis) by the enzyme phosphoglucomutase. Glucose residues are phosphorolysed from branches of glycogen until four residues before a glucose that is branched with a α →6linkage. Glycogen debranching enzyme then transfers three of the remaining four glucose units to the end of another glycogen branch. This exposes the α →6branching point, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Sugar Level
Glycaemia, also known as blood sugar level, blood sugar concentration, or blood glucose level is the measure of glucose concentrated in the blood of humans or other animals. Approximately 4 grams of glucose, a simple sugar, is present in the blood of a 70 kg (154 lb) human at all times. The body tightly regulates blood glucose levels as a part of metabolic homeostasis. Glucose is stored in skeletal muscle and liver cells in the form of glycogen; in fasting individuals, blood glucose is maintained at a constant level at the expense of glycogen stores in the liver and skeletal muscle. In humans, a blood glucose level of 4 grams, or about a teaspoon, is critical for normal function in a number of tissues, and the human brain consumes approximately 60% of blood glucose in fasting, sedentary individuals. A persistent elevation in blood glucose leads to glucose toxicity, which contributes to cell dysfunction and the pathology grouped together as complications of diabetes. Gl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



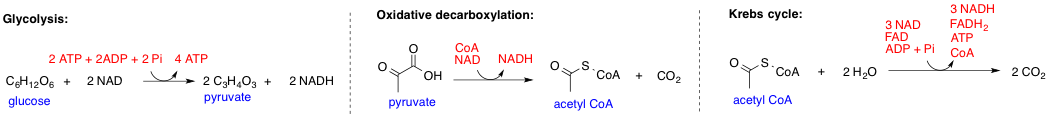

.jpg)
