|
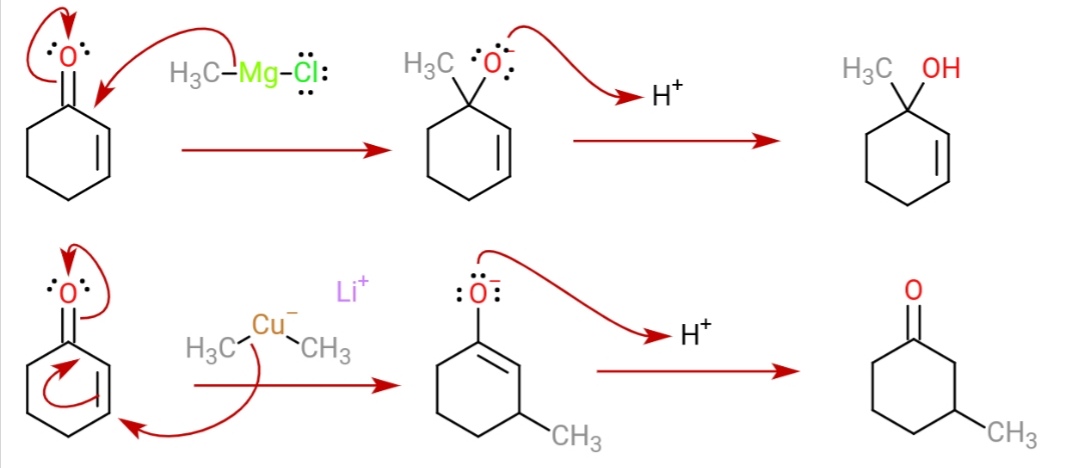
Gilman Reagent
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Reactions These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. : Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilman Reagent2
Gilman may refer to: Places United States *Gilman Ranch, California *Gilman, Colorado *Gilman, Illinois *Gilman, Iowa * Gilman, Minnesota *Gilman, Montana *Gilman, Vermont * Gilman, Washington, former name of Issaquah *Gilman, Pierce County, Wisconsin *Gilman, Taylor County, Wisconsin *Gilman Lake, a lake in South Dakota *Gilmanton, New Hampshire *Gilmanton, Buffalo County, Wisconsin *Gilmanton Township, Benton County, Minnesota Other *Gilman (Yap), an administrative division of the Federated States of Micronesia *Gilman Street, a street in Central, Hong Kong Other uses *Gilman (name) *Gilman reagent, any of a group of reagents discovered by Henry Gilman *Gilman Paper Company, former paper producer ** Gilman Paper Company collection, photo archive in the Metropolitan Museum of Art *Gilman School, a private boys school in Baltimore, Maryland *924 Gilman Street The Alternative Music Foundation located at 924 Gilman Street, often referred to by its fans simply as "Gilman", i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Organic Chemistry
''The Journal of Organic Chemistry'', colloquially known as ''JOC'', is a peer-reviewed scientific journal for original contributions of fundamental research in all branches of theory and practice in organic and bioorganic chemistry. It is published by the publishing arm of the American Chemical Society, with 24 issues per year. According to the ''Journal Citation Reports'', the journal had a 2017 impact factor of 4.805 and it is the journal that received the most cites (100,091 in 2017) in the field of organic chemistry. According to Web of Knowledge (and as December 2012), eleven papers from the journal have received more than 1,000 citations, with the most cited paper having received 7,967 citations. The current editor-in-chief is Scott J. Miller from Yale University. Indexing ''J. Org. Chem.'' is currently indexed in: See also *Organic Letters *Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angewandte Chemie
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2021 impact factor of 16.823. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'' ( (print), (online)), and a fully English-language edition, ''Angewandte Chemie International Edition'' ( (print), (online)). The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Business model ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilman Vs Grignard
Gilman may refer to: Places United States *Gilman Ranch, California *Gilman, Colorado *Gilman, Illinois *Gilman, Iowa * Gilman, Minnesota *Gilman, Montana *Gilman, Vermont * Gilman, Washington, former name of Issaquah *Gilman, Pierce County, Wisconsin *Gilman, Taylor County, Wisconsin *Gilman Lake, a lake in South Dakota *Gilmanton, New Hampshire *Gilmanton, Buffalo County, Wisconsin *Gilmanton Township, Benton County, Minnesota Other *Gilman (Yap), an administrative division of the Federated States of Micronesia *Gilman Street, a street in Central, Hong Kong Other uses *Gilman (name) *Gilman reagent, any of a group of reagents discovered by Henry Gilman *Gilman Paper Company, former paper producer ** Gilman Paper Company collection, photo archive in the Metropolitan Museum of Art *Gilman School, a private boys school in Baltimore, Maryland *924 Gilman Street The Alternative Music Foundation located at 924 Gilman Street, often referred to by its fans simply as "Gilman", i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilman Reaction Example
Gilman may refer to: Places United States *Gilman Ranch, California *Gilman, Colorado *Gilman, Illinois *Gilman, Iowa * Gilman, Minnesota *Gilman, Montana *Gilman, Vermont * Gilman, Washington, former name of Issaquah *Gilman, Pierce County, Wisconsin *Gilman, Taylor County, Wisconsin *Gilman Lake, a lake in South Dakota *Gilmanton, New Hampshire *Gilmanton, Buffalo County, Wisconsin *Gilmanton Township, Benton County, Minnesota Other *Gilman (Yap), an administrative division of the Federated States of Micronesia *Gilman Street, a street in Central, Hong Kong Other uses *Gilman (name) *Gilman reagent, any of a group of reagents discovered by Henry Gilman *Gilman Paper Company, former paper producer ** Gilman Paper Company collection, photo archive in the Metropolitan Museum of Art *Gilman School, a private boys school in Baltimore, Maryland *924 Gilman Street The Alternative Music Foundation located at 924 Gilman Street, often referred to by its fans simply as "Gilman", i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-beta Unsaturated Carbonyl Compounds
Alphabeta is an Israeli musical group. Alphabeta or Alpha Beta may also refer to: *The Greek alphabet, from ''Alpha'' (Αα) and ''Beta'' (Ββ), the first two letters *Alpha Beta, a former chain of Californian supermarkets *Alpha and beta anomers (chemistry) *Alpha–beta pruning, a type of search algorithm *Alpha-beta transformation, a mathematical transformation in electrical engineering *Alpha-beta unsaturated carbonyl compounds, a class of organic compounds *Alpha beta filter, a predictive filter *Alpha (finance) and Beta (finance), two measures characterizing the return of an investment portfolio *The Alpha Betas, a fraternity in the ''Revenge of the Nerds ''Revenge of the Nerds'' is a 1984 American comedy film directed by Jeff Kanew and starring Robert Carradine, Anthony Edwards, Ted McGinley, and Bernie Casey. The film's plot chronicles a group of nerds at the fictional Adams College trying ...'' film series *''Alpha Betas,'' an animated webseries created by Chris B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Acyl Substitution
Nucleophilic acyl substitution describe a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products. Reaction mechanism Carbonyl compounds react with nucleophiles via an addition mechanism: the nucleophile attacks the carbonyl carbon, forming a tetrahedral intermediate. This reaction can be accelerated by acidic conditions, which make the carbonyl more electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles. Reaction mechanism Conjugate addition is the vinylogous counterpart of direct nucleophilic addition. A nucleophile reacts with a α,β-unsaturated carbonyl compound in the β position. The negative charge carried by the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |