|
Gastric Fluid
Gastric acid, gastric juice, or stomach acid is a digestive fluid formed within the stomach lining. With a pH between 1 and 3, gastric acid plays a key role in digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract. The active components of gastric acid are protons and chloride. Often simplistically described as hydrochloric acid, these species are produced by parietal cells in the gastric glands in the stomach. The secre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastric Mucosa
The gastric mucosa is the mucous membrane layer of the stomach, which contains the glands and the gastric pits. In humans, it is about 1 mm thick, and its surface is smooth, soft, and velvety. It consists of simple columnar epithelium, lamina propria, and the muscularis mucosae. Description In its fresh state, it is of a pinkish tinge at the pyloric end and of a red or reddish-brown color over the rest of its surface. In infancy it is of a brighter hue, the vascular redness being more marked. It is thin at the cardiac extremity, but thicker toward the pylorus. During the contracted state of the organ it is thrown into numerous plaits or rugae, which, for the most part, have a longitudinal direction, and are most marked toward the pyloric end of the stomach, and along the greater curvature. These folds are entirely obliterated when the organ becomes distended. When examined with a lens, the inner surface of the mucous membrane presents a peculiar honeycomb appearance fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lumen (anatomy)
In biology, a lumen (plural lumina) is the inside space of a tubular structure, such as an artery or intestine. It comes . It can refer to: *The interior of a vessel, such as the central space in an artery, vein or capillary through which blood flows. *The interior of the gastrointestinal tract *The pathways of the bronchi in the lungs *The interior of renal tubules and urinary collecting ducts *The pathways of the female genital tract, starting with a single pathway of the vagina, splitting up in two lumina in the uterus, both of which continue through the Fallopian tubes In cell biology, a lumen is a membrane-defined space that is found inside several organelles, cellular components, or structures: *thylakoid, endoplasmic reticulum, Golgi apparatus, lysosome, mitochondrion, or microtubule Transluminal procedures ''Transluminal procedures'' are procedures occurring through lumina, including: *Natural orifice transluminal endoscopic surgery in the lumina of, for example, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
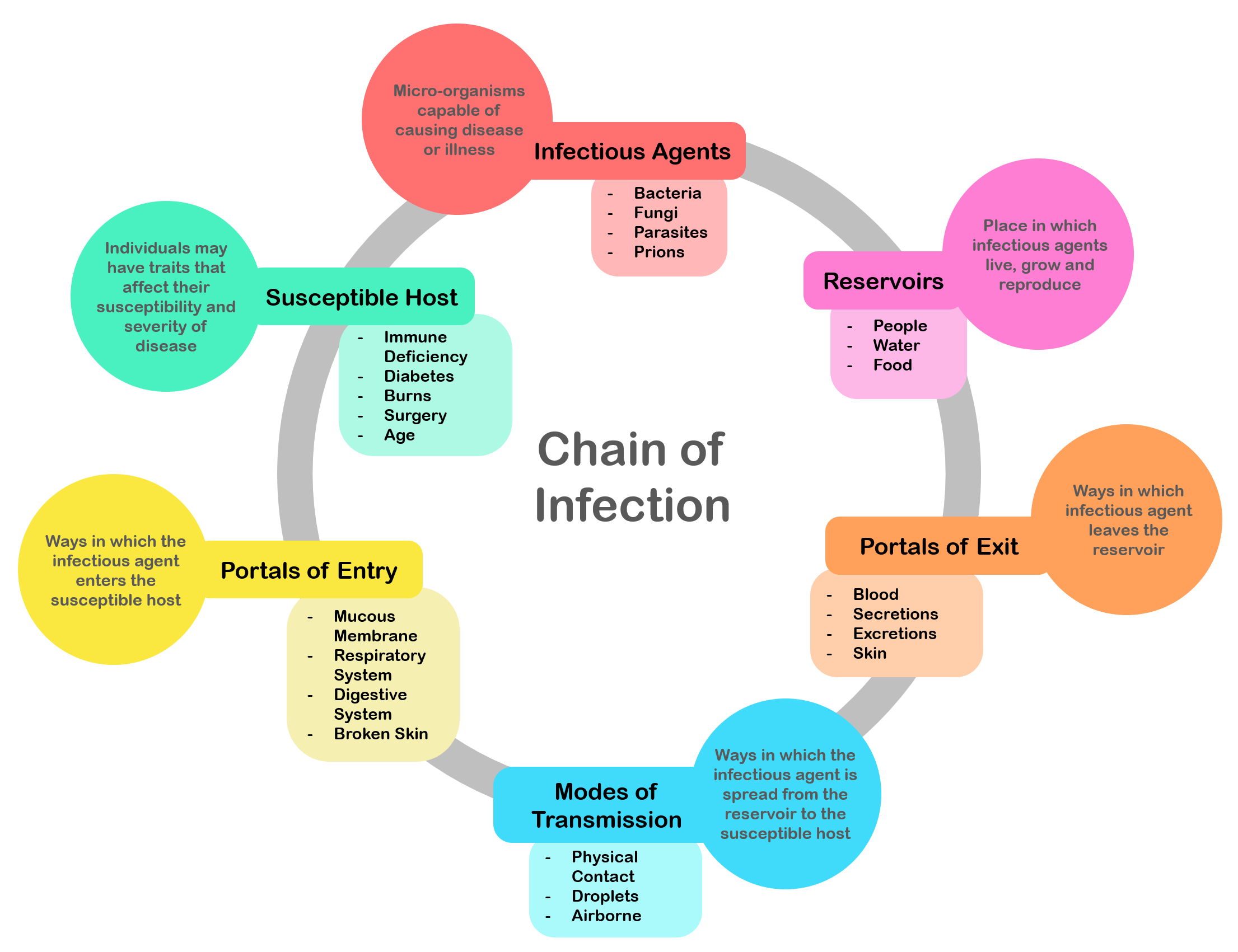
Infection
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable disease, is an illness resulting from an infection. Infections can be caused by a wide range of pathogens, most prominently bacteria and viruses. Hosts can fight infections using their immune system. Mammalian hosts react to infections with an innate response, often involving inflammation, followed by an adaptive response. Specific medications used to treat infections include antibiotics, antivirals, antifungals, antiprotozoals, and antihelminthics. Infectious diseases resulted in 9.2 million deaths in 2013 (about 17% of all deaths). The branch of medicine that focuses on infections is referred to as infectious disease. Types Infections are caused by infectious agents (pathogens) including: * Bacteria (e.g. ''Mycobacterium tuberculosis'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in older texts. The informal synonym ''microbe'' () comes from μικρός, mikrós, "small" and βίος, bíos, "life". is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from ancient times, such as in Jain scriptures from sixth century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Because mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pepsin
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site. It is one of three principal endopeptidases (enzymes cutting proteins in the middle) in the human digestive system, the other two being chymotrypsin and trypsin. There are also exopeptidases which remove individual amino acids at both ends of proteins (carboxypeptidases produced by the pancreas and aminopeptidases secreted by the small intestine). During the process of digestion, these enzymes, each of which is specialized in severing links between particular types of amino acids, collaborate to break down dietary proteins into their components, i.e., peptides and amino acids, which can be readily absorbed by the sma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rennin
Chymosin or rennin is a protease found in rennet. It is an aspartic endopeptidase belonging to MEROPS A1 family. It is produced by newborn ruminant animals in the lining of the abomasum to curdle the milk they ingest, allowing a longer residence in the bowels and better absorption. It is widely used in the production of cheese. Bovine chymosin is now produced recombinantly in , '' Aspergillus niger var awamori'', and as alternative resource. Occurrence The chymosin is found in a wide range of tetrapods, although it is best known to be produced by ruminant animals in the lining of the abomasum. Chymosin is produced by gastric chief cells in newborn mammals to curdle the milk they ingest, allowing a longer residence in the bowels and better absorption. Non-ruminant species that produce chymosin include pigs, cats, seals,Staff, Online Mendelian Inheritance in Man (OMIM) Database. Last updated February 21, 199Chymosin pseudogene; CYMP prochymosin, included, in the OMIM/ref> a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pepsinogen
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site. It is one of three principal endopeptidases (enzymes cutting proteins in the middle) in the human digestive system, the other two being chymotrypsin and trypsin. There are also exopeptidases which remove individual amino acids at both ends of proteins (carboxypeptidases produced by the pancreas and aminopeptidases secreted by the small intestine). During the process of digestion, these enzymes, each of which is specialized in severing links between particular types of amino acids, collaborate to break down dietary proteins into their components, i.e., peptides and amino acids, which can be readily absorbed by the small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastric Chief Cell
A gastric chief cell (or peptic cell, or gastric zymogenic cell) is a type of gastric gland cell that releases pepsinogen and gastric lipase. It is the cell responsible for secretion of chymosin in ruminant animals. The cell stains basophilic upon H&E staining due to the large proportion of rough endoplasmic reticulum in its cytoplasm. Gastric chief cells are generally located deep in the mucosal layer of the stomach lining, in the fundus and body of the stomach.pathologyoutlines.com/topic/stomachnormalhistology.html Chief cells release the zymogen (enzyme precursor) pepsinogen when stimulated by a variety of factors including cholinergic activity from the vagus nerve and acidic condition in the stomach. Gastrin and secretin may also act as secretagogues. It works in conjunction with the parietal cell, which releases gastric acid, converting the pepsinogen into pepsin. Nomenclature The terms ''chief cell'' and ''zymogenic cell'' are often used without the word "gastric" to n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
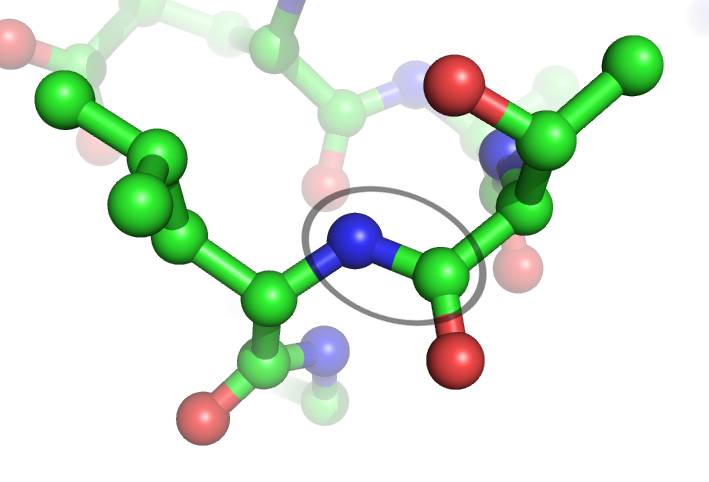
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a ''dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |