|
Gamma Ray Cross Section
Gamma ray cross section - a measure of the probability that gamma ray interacts with matter. The total cross section of gamma ray interactions is composed of several independent processes: photoelectric effect, Compton scattering, electron-positron pair production in the nucleus field and electron-positron pair production in the electron field (triplet production). The cross section for single process listed above is a part of the total gamma ray cross section. Other effects, like the photonuclear absorption, Thomson or Rayleigh (coherent) scattering can be omitted because of their nonsignificant contribution in the gamma ray range of energies. The detailed equations for cross sections (barn/atom) of all mentioned effects connected with gamma ray interaction with matter are listed below. Photoelectric effect cross section This phenomenon describes the situation in which a gamma photon interacts with an electron located in the atomic structure. This results the ejection of that e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fine-structure Constant
In physics, the fine-structure constant, also known as the Sommerfeld constant, commonly denoted by (the Greek letter ''alpha''), is a fundamental physical constant which quantifies the strength of the electromagnetic interaction between elementary charged particles. It is a dimensionless quantity, independent of the system of units used, which is related to the strength of the coupling of an elementary charge ''e'' with the electromagnetic field, by the formula . Its numerical value is approximately , with a relative uncertainty of The constant was named by Arnold Sommerfeld, who introduced it in 1916 Equation 12a, ''"rund 7·" (about ...)'' when extending the Bohr model of the atom. quantified the gap in the fine structure of the spectral lines of the hydrogen atom, which had been measured precisely by Michelson and Morley in 1887. Definition In terms of other fundamental physical constants, may be defined as: \alpha = \frac = \frac , where * is the elementary char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attenuation Coefficient
The linear attenuation coefficient, attenuation coefficient, or narrow-beam attenuation coefficient characterizes how easily a volume of material can be penetrated by a beam of light, sound, particles, or other energy or matter. A coefficient value that is large represents a beam becoming 'attenuated' as it passes through a given medium, while a small value represents that the medium had little effect on loss. The SI unit of attenuation coefficient is the reciprocal metre (m−1). Extinction coefficient is another term for this quantity, often used in meteorology and climatology. Most commonly, the quantity measures the exponential decay of intensity, that is, the value of downward ''e''-folding distance of the original intensity as the energy of the intensity passes through a unit (''e.g.'' one meter) thickness of material, so that an attenuation coefficient of 1 m−1 means that after passing through 1 metre, the radiation will be reduced by a factor of '' e'', and for material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beer–Lambert Law
The Beer–Lambert law, also known as Beer's law, the Lambert–Beer law, or the Beer–Lambert–Bouguer law relates the attenuation of light to the properties of the material through which the light is travelling. The law is commonly applied to chemical analysis measurements and used in understanding attenuation in physical optics, for photons, neutrons, or rarefied gases. In mathematical physics, this law arises as a solution of the BGK equation. History The law was discovered by Pierre Bouguer before 1729, while looking at red wine, during a brief vacation in Alentejo, Portugal. It is often attributed to Johann Heinrich Lambert, who cited Bouguer's ''Essai d'optique sur la gradation de la lumière'' (Claude Jombert, Paris, 1729) – and even quoted from it – in his ''Photometria'' in 1760. Lambert's law stated that the loss of light intensity when it propagates in a medium is directly proportional to intensity and path length. Much later, the German scientist Augus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pair Production
Pair production is the creation of a subatomic particle and its antiparticle from a neutral boson. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton. Pair production often refers specifically to a photon creating an electron–positron pair near a nucleus. As energy must be conserved, for pair production to occur, the incoming energy of the photon must be above a threshold of at least the total rest mass energy of the two particles created. (As the electron is the lightest, hence, lowest mass/energy, elementary particle, it requires the least energetic photons of all possible pair-production processes.) Conservation of energy and momentum are the principal constraints on the process. All other conserved quantum numbers (angular momentum, electric charge, lepton number) of the produced particles must sum to zero thus the created particles shall have opposite values of each other. For instance, if one particle has electric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riemann Zeta Function
The Riemann zeta function or Euler–Riemann zeta function, denoted by the Greek letter (zeta), is a mathematical function of a complex variable defined as \zeta(s) = \sum_^\infty \frac = \frac + \frac + \frac + \cdots for \operatorname(s) > 1 and its analytic continuation elsewhere. The Riemann zeta function plays a pivotal role in analytic number theory, and has applications in physics, probability theory, and applied statistics. Leonhard Euler first introduced and studied the function over the reals in the first half of the eighteenth century. Bernhard Riemann's 1859 article "On the Number of Primes Less Than a Given Magnitude" extended the Euler definition to a complex variable, proved its meromorphic continuation and functional equation, and established a relation between its zeros and the distribution of prime numbers. This paper also contained the Riemann hypothesis, a conjecture about the distribution of complex zeros of the Riemann zeta function that is consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collides with an electron, annihilation occurs. If this collision occurs at low energies, it results in the production of two or more photons. Positrons can be created by positron emission radioactive decay (through weak interactions), or by pair production from a sufficiently energetic photon which is interacting with an atom in a material. History Theory In 1928, Paul Dirac published a paper proposing that electrons can have both a positive and negative charge. This paper introduced the Dirac equation, a unification of quantum mechanics, special relativity, and the then-new concept of electron spin to explain the Zeeman effect. The paper did not explicitly predict a new particle but did allow for electrons having either positive or negative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium atomic radiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
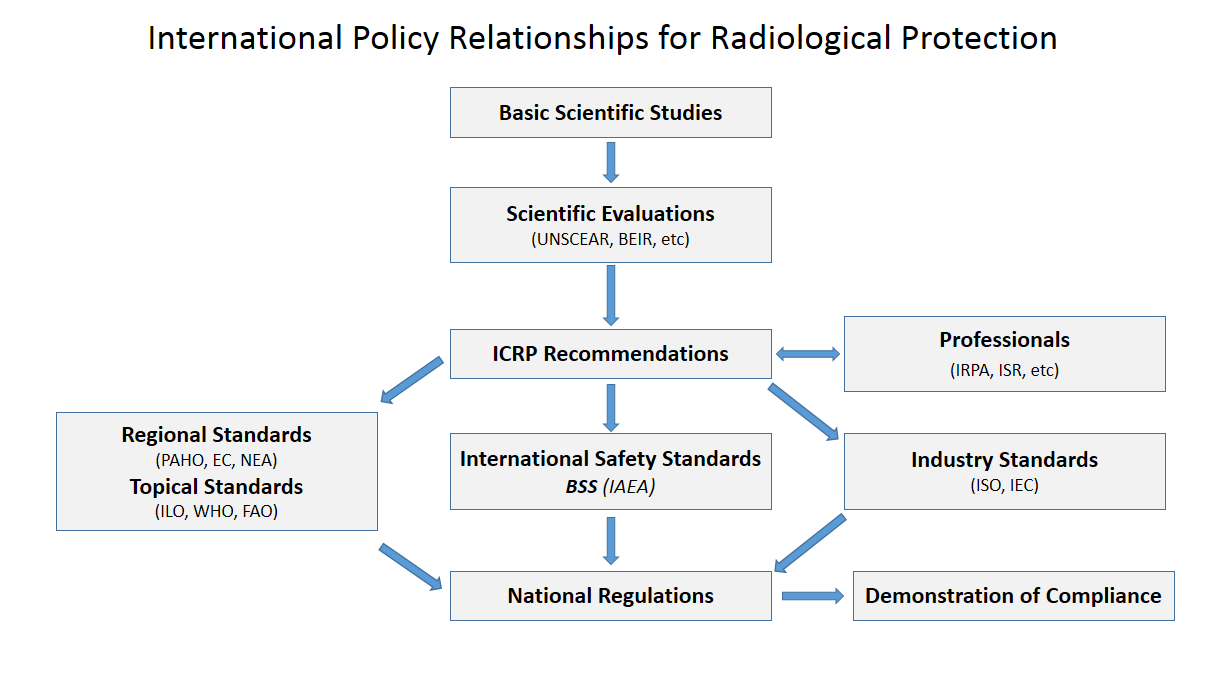
Radiation Protection
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination. Ionizing radiation is widely used in industry and medicine, and can present a significant health hazard by causing microscopic damage to living tissue. There are two main categories of ionizing radiation health effects. At high exposures, it can cause "tissue" effects, also called "deterministic" effects due to the certainty of them happening, conventionally indicated by the unit gray and resulting in acute radiation syndrome. For low level exposures there can be statistically elevated risks of radiation-induced cancer, called " stochastic effects" due to the uncertainty of them happening, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klein–Nishina Formula
In particle physics, the Klein–Nishina formula gives the differential cross section (i.e. the "likelihood" and angular distribution) of photons scattered from a single free electron, calculated in the lowest order of quantum electrodynamics. It was first derived in 1928 by Oskar Klein and Yoshio Nishina, constituting one of the first successful applications of the Dirac equation. The formula describes both the Thomson scattering of low energy photons (e.g. visible light) and the Compton scattering of high energy photons (e.g. x-rays and gamma-rays), showing that the total cross section and expected deflection angle decrease with increasing photon energy. Formula For an incident unpolarized photon of energy E_\gamma, the differential cross section is: : \frac = \frac r_e^2 \left(\frac\right)^ \left frac + \frac - \sin^2(\theta)\right where * r_e is the classical electron radius (~2.82 fm, r_e^2 is about 7.94 × 10−30 m2 or 79.4 mb) * \lambda/\lambda' is the ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (electromagnetic Radiation)
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect is attenuation, or the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning synonymous with "absorption coefficient") * The Molar attenuation coefficient (a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |