|
Gallium Insurance Co
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan (62–� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environmentally Friendly
Environment friendly processes, or environmental-friendly processes (also referred to as eco-friendly, nature-friendly, and green), are sustainability and marketing terms referring to goods and services, laws, guidelines and policies that claim reduced, minimal, or no harm upon ecosystems or the environment. Companies use these ambiguous terms to promote goods and services, sometimes with additional, more specific certifications, such as ecolabels. Their overuse can be referred to as greenwashing.Greenwashing Fact Sheet. 22 March 2001. Retrieved 14 November 2009. frocorpwatch.org/ref> To ensure the successful meeting of Sustainable Development Goals (SDGs) companies are advised to employ environmental friendly processes in their production. Specifically, Sustainable Development Goal 12 measures 11 targets and 13 indicators "to ensure sustainable consumption and production patterns". The International Organization for Standardization has developed ISO 14020 and ISO 14024 to es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
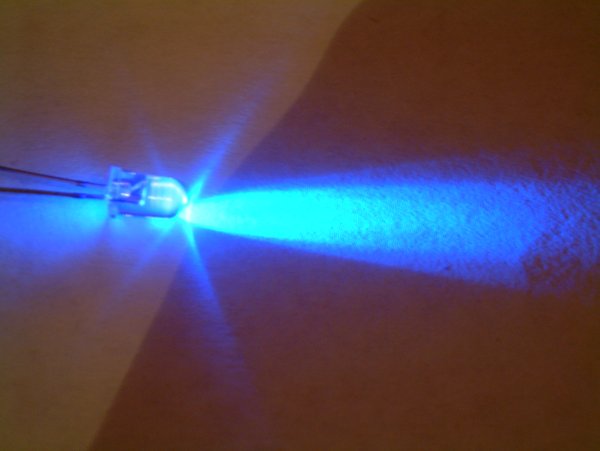
Light-emitting Diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device. Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared (IR) light. Infrared LEDs are used in remote-control circuits, such as those used with a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Early LEDs were often used as indicator lamps, replacing small incandescent bulbs, and in seven-segment displays. Later developments produced LEDs available in visible, ultraviolet (UV) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Gallium Nitride
Indium gallium nitride (InGaN, ) is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared (0.69 eV) for InN to the ultraviolet (3.4 eV) of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7. Applications LEDs Indium gallium nitride is the light-emitting layer in modern blue and green LEDs and often grown on a GaN buffer on a transparent substrate as, e.g. sapphire or silicon carbide. It has a high heat capacity and its sensitivity to ionizing radiation is low (like other group III nitrides), making it also a potentially suitable material for solar photovoltaic devices, specifically for arrays for satellites. It is theoretically predicted that spinodal decomposition of indium nitride should occur for compositions between 15% and 85%, leadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Nitride
Gallium nitride () is a binary III/ V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic, high-power and high-frequency devices. For example, GaN is the substrate which makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency-doubling. Its sensitivity to ionizing radiation is low (like other group III nitrides), making it a suitable material for solar cell arrays for satellites. Military and space applications could also benefit as devices have shown stability in high radiation environments. Because GaN transistors can operate at much higher temperatures and work at much higher voltages than gallium arsenide (GaAs) transistors, they make ideal power amplifiers at microwave frequencies. In addition, GaN offers promising characteris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ranges as microwaves; the above broad definition includes both UHF and EHF (millimeter wave) bands. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 0.3 m and 3 mm). In all cases, microwaves include the entire SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum. Frequencies in the microwave range are often referred to by their IEEE radar band designations: S, C, X, Ku, K, or Ka band, or by similar NATO or EU designations. The prefix ' in ''microwave'' is not meant to suggest a wavelength in the micrometer range. Rather, it indicates that microwaves are "small" (having shorter wavelengths), compared to the radio waves used prior to microwave te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. Preparation and chemistry In the compound, gallium has a +3 oxidation state. Gallium arsenide single crystals can be prepared by three industrial processes: * The vertical gradient freeze (VGF) process. * Crystal growth using a horizontal zone furnace in the Bridgman-Stockbarger technique, in which gallium and arsenic vapors react, and free molecules deposit on a seed crystal at the cooler end of the furnace. * Liquid encapsulated Czochralski process, Czoch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronics
The field of electronics is a branch of physics and electrical engineering that deals with the emission, behaviour and effects of electrons using electronic devices. Electronics uses active devices to control electron flow by amplification and rectification, which distinguishes it from classical electrical engineering, which only uses passive effects such as resistance, capacitance and inductance to control electric current flow. Electronics has hugely influenced the development of modern society. The central driving force behind the entire electronics industry is the semiconductor industry sector, which has annual sales of over $481 billion as of 2018. The largest industry sector is e-commerce, which generated over $29 trillion in 2017. History and development Electronics has hugely influenced the development of modern society. The identification of the electron in 1897, along with the subsequent invention of the vacuum tube which could amplify and rectify small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bauxite
Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)), mixed with the two iron oxides goethite (FeO(OH)) and haematite (Fe2O3), the aluminium clay mineral kaolinite (Al2Si2O5(OH)4) and small amounts of anatase (TiO2) and ilmenite (FeTiO3 or FeO.TiO2). Bauxite appears dull in luster and is reddish-brown, white, or tan. In 1821, the French geologist Pierre Berthier discovered bauxite near the village of Les Baux in Provence, southern France. Formation Numerous classification schemes have been proposed for bauxite but, , there was no consensus. Vadász (1951) distinguished lateritic bauxites (silicate bauxites) from karst bauxite ores (carbonate bauxites): * The carbonate bauxites occur predominantly in Europe, Guyana, Suriname, and Jamaica above carbonate rocks (limestone and do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hosted lead-zinc ore deposits, Mississippi-Valley type, and Volcanogenic massive sulfide ore deposit, volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfide mineral, sulfides), calcite, dolomite (mineral), dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
%2C_black_and_white.png)