|
Galidesivir
Galidesivir (BCX4430, immucillin-A) is an antiviral drug, an adenosine analog (a type of nucleoside analog). It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under Phases of clinical research, phase 1 human trial in Brazil for coronavirus. It also shows Broad-spectrum antiviral drug, broad-spectrum antiviral effectiveness against a range of other RNA virus families, including bunyaviruses, arenaviruses, Paramyxoviridae, paramyxoviruses, coronaviruses, flaviviruses, and phleboviruses. Galidesivir has been demonstrated to protect against both Ebola and Marburg viruses in both rodents and monkeys, even when administered up to 48 hours after infection, and development for use in humans was then being fast-tracked due to concerns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BioCryst Pharmaceuticals
BioCryst Pharmaceuticals, Inc. is an American pharmaceutical company headquartered in Durham, North Carolina. The company is a late stage biotech company that focuses on oral drugs for rare and serious diseases. BioCryst's antiviral drug peramivir (Rapivab) was approved by FDA in December 2014. It has also been approved in Japan, Korea, and China. History The company was founded in 1986 by Charles E. Bugg and John A. Montgomery. In March 1994, BioCryst became a public company when it completed an initial public offering by listing its shares on the NASDAQ stock exchange. In 2008, the company was named one of the fastest growing companies by Deloitte & Touche in its 2008 list of ''Technology Fast 500''. In October 2010, BioCryst announced its headquarters would move to Durham, North Carolina, where the company has had an office since 2006. In January 2018, BioCryst signed a definitive merger agreement with Idera Pharmaceuticals, with plans for the combined company to change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
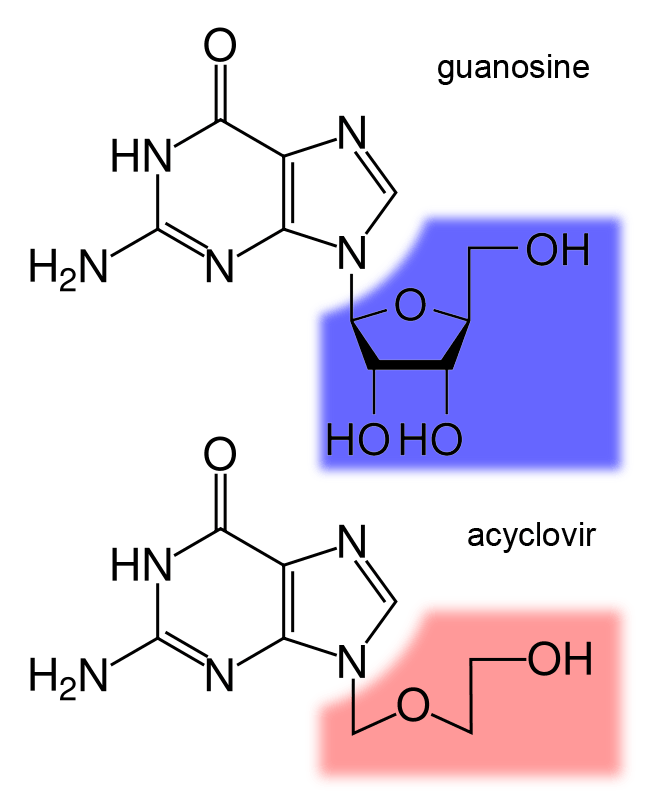
Nucleoside Analog
Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates. Nucleoside and nucleotide analogues can be used in therapeutic drugs, including a range of antiviral products used to prevent viral replication in infected cells. The most commonly used is acyclovir, although its inclusion in this category is uncertain, because it acts as a nucleoside but contains no actual sugar, as the sugar ring is replaced by an open-chain structure. Nucleotide and nucleoside analogues can also be found naturally. Examples include ddhCTP (3ʹ-deoxy-3′,4ʹdidehydro-CTP) produced by the human antiviral protein viperin and sinefungin (a S-Adenosyl methionine analogue) produced by some ''Streptomyces''. Function These agents can be used against hepatitis B virus, hepatitis C virus, herpes simplex, and HIV. Once they are phosphorylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiviral Drug
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic (also termed antibacterial), antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees. Medical uses Most of the antiviral drugs now available are designed to help deal with HIV, herpes viruses, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ebola Virus Epidemic In West Africa
The 2013–2016 epidemic of Ebola virus disease, centered in West Africa, Western Africa, was the most widespread List of Ebola outbreaks, outbreak of the disease in history. It caused major loss of life and Socioeconomics, socioeconomic disruption in the region, mainly in Ebola virus epidemic in Guinea, Guinea, Ebola virus epidemic in Liberia, Liberia and Ebola virus epidemic in Sierra Leone, Sierra Leone. The first cases were recorded in Guinea in December 2013; later, the disease spread to neighbouring Liberia and Sierra Leone, with minor outbreaks occurring in Ebola virus disease in Nigeria, Nigeria and Ebola virus disease in Mali, Mali. Secondary infections of medical workers occurred in the Ebola virus cases in the United States, United States and Ebola virus disease in Spain, Spain. In addition, isolated cases were recorded in Senegal, the Ebola virus disease in the United Kingdom, United Kingdom and Italy. The number of cases peaked in October 2014 and then began to de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FGI-106
FGI-106 is a broad-spectrum antiviral drug developed as a potential treatment for enveloped RNA viruses, in particular viral hemorrhagic fevers from the bunyavirus, flavivirus and filovirus families. It acts as an inhibitor which blocks viral entry into host cells. In animal tests FGI-106 shows both prophylactic and curative action against a range of deadly viruses for which few existing treatments are available, including the bunyaviruses hantavirus, Rift Valley fever virus and Crimean-Congo hemorrhagic fever virus, the flavivirus dengue virus, and the filoviruses Ebola virus and Marburg virus. See also * Brincidofovir * BCX4430 * Favipiravir * FGI-103 * FGI-104 * LJ-001 * TKM-Ebola * ZMapp ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada ... References Anti–RNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Favipiravir
Favipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan. It is also being studied to treat a number of other viral infections, including SARS-CoV-2. Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative. It is being developed and manufactured by Toyama Chemical (a subsidiary of Fujifilm) and was approved for medical use in Japan in 2014. In 2016, Fujifilm licensed it to Zhejiang Hisun Pharmaceutical Co. It became a generic drug in 2019. Medical use Favipiravir has been approved to treat influenza in Japan. It is, however, only indicated for novel influenza (strains that cause more severe disease) rather than seasonal influenza. As of 2020, the probability of resistance developing appears low. Side effects There is evidence that use during pregnancy may result in harm to the baby. Teratogenic and embryotoxic effects were shown on four animal species. Mechanism of actio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DZNep
3-Deazaneplanocin A (DZNep, C-c3Ado) is a drug which acts as both a ''S''-adenosylhomocysteine synthesis inhibitor and also a histone methyltransferase EZH2 inhibitor. Studies have shown that it has ''in vitro'' against a variety of different tumor cell lines. In studies on mice, the drug was also found to be effective for the treatment of Ebola virus disease, apparently interfering with the Ebola viruses ability to block interferon Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten the ... production, thus restoring the ability of immune system to rid the body of ebolavirus. References {{DEFAULTSORT:Deazaneplanocin A, 3- Nucleosides Imidazopyridines Polyols Experimental drugs Cyclopentenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronavir
Coronavir is an anti-viral drug approved in Russia for the treatment of COVID-19. Little is known of this drug, except for the information released by its developer R-Pharm. By the description provided to the press, coronavir seems to be an inhibitor of SARS-CoV-2 RNA polymerase, similar to other antiviral nucleotide analogues such as remdesivir. The drug appears to be based on favipiravir, a drug developed in Japan. Coronavir was approved for use in Russia in hospitals in July 2020, and in September 2020 it received approval for prescription sales for outpatient use. See also * COVID-19 pandemic in Russia The COVID-19 pandemic in Russia is part of the ongoing pandemic of coronavirus disease 2019 () caused by severe acute respiratory syndrome coronavirus 2 (). The virus was confirmed to have spread to Russia on 31 January 2020, when two Ch ... References COVID-19 pandemic in Russia Antiviral drugs Drugs with undisclosed chemical structures {{COVID-19-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brincidofovir
Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability. The most common side effects include diarrhea, nausea, vomiting, and abdominal pain. Brincidofovir was approved for medical use in the United States in June 2021. Medical uses Brincidofovir is indicated for the treatment of human smallpox disease caused by variola virus. Mechanism of Action Brincidofovir is a pro-drug that is composed of cidofovir, conjugated with a lipid molecule. The lipid aspect of the molecule takes on the action of endogenous lysopghosphatidyl choline, which then is able to enter cells in the body which are infected with smallpox. Once the infected cell takes in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bemnifosbuvir
Bemnifosbuvir (AT-527, RO7496998) is an antiviral drug invented by Atea Pharmaceuticals and licensed to Roche for clinical development, a novel nucleotide analog prodrug originally developed for the treatment of hepatitis C. AT-527 is the orally bioavailable hemisulfate salt of AT-511, which is metabolised in several steps to the active nucleotide triphosphate AT-9010, acting as an RNA polymerase inhibitor and thereby interfering with viral replication. AT-527 has been researched for the treatment of coronavirus diseases such as that produced by SARS-CoV-2. It showed good results in early clinical trials but had inconsistent results at later stages, so the planned Phase 3 trials are being redesigned and results are not expected until late 2022. See also * Galidesivir * Remdesivir * Lufotrelvir * Molnupiravir * Sofosbuvir Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken Oral administration, by mouth. Common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
