|
Gadolinium(III) Nitrate
Gadolinium(III) nitrate is an inorganic compound of gadolinium. This salt is used as a water-soluble neutron poison in nuclear reactors. Gadolinium nitrate, like all nitrate salts, is an oxidizing agent. The most common form of this substance is hexahydrate Gd(NO3)3•6H2O with molecular weight 451.36 g/mol and CAS Number: 19598-90- Use Gadolinium nitrate was used at the Savannah River Site heavy water nuclear reactors and had to be separated from the heavy water for storage or reuse. The Canadian CANDU reactor, a pressurized heavy water reactor, also uses gadolinium nitrate as a water-soluble neutron poison in heavy water. Gadolinium nitrate is also used as a raw material in the production of other gadolinium compounds, for production of specialty glasses and ceramics and as a phosphor A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties. Gadolinium was discovered in 1880 by Jean Charles de Marignac, who detected its oxide by using spectroscopy. It is named after the mineral gadolinite, one of the minerals in which gadolinium is found, itself named for the Finnish chemist Johan Gadolin. Pure gadolinium was first isolated by the chemist Paul-Émile Lecoq de Boisbaudran around 1886. Gadoliniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant. The capture of neutrons by short half-life fission products is known as reactor poisoning; neutron capture by long-lived or stable fission products is called reactor slagging. Transient fission product poisons Some of the fission products generated during nuclear reactions have a high neutron absorption capacity, such as xenon-135 (microscopic cross-section σ = 2,000,000 barns (b); up to 3 million barns in reactor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid (water or gas), which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. , the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world. In the early era of nuclear reactors (1940s), a reactor was known as a nuclear pile or atomic pile (so-called because the graphite moderator blocks of the first reactor were placed into a tall pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
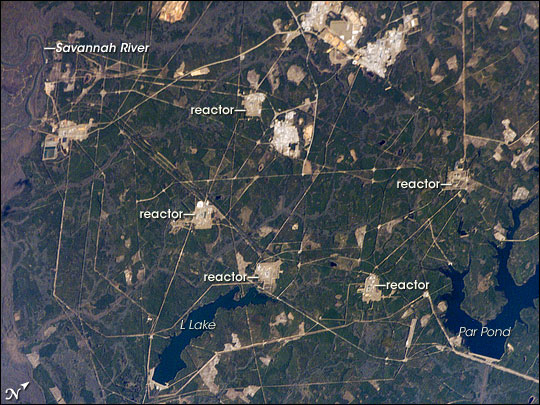
Savannah River Site
The Savannah River Site (SRS) is a U.S. Department of Energy (DOE) reservation in the United States in the state of South Carolina, located on land in Aiken, Allendale, and Barnwell counties adjacent to the Savannah River, southeast of Augusta, Georgia. The site was built during the 1950s to refine nuclear materials for deployment in nuclear weapons. It covers and employs more than 10,000 people. It is owned by the U.S. Department of Energy (DOE). The management and operating contract is held by Savannah River Nuclear Solutions LLC ( SRNS), a partnership between Fluor Corporation, Newport News Nuclear, Inc. (a subsidiary of Huntington Ingalls Industries) and Honeywell International, and the Integrated Mission Completion contract (including the former scope of the Liquid Waste Operations contract) is held by Savannah River Mission Completion, which is a team of companies led by BWX Technologies, AECOM, and Fluor. A major focus is cleanup activities related to work done in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CANDU Reactor
The CANDU (Canada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide ( heavy water) moderator and its use of (originally, natural) uranium fuel. CANDU reactors were first developed in the late 1950s and 1960s by a partnership between Atomic Energy of Canada Limited (AECL), the Hydro-Electric Power Commission of Ontario, Canadian General Electric, and other companies. There have been two major types of CANDU reactors, the original design of around 500 MWe that was intended to be used in multi-reactor installations in large plants, and the rationalized CANDU 6 in the 600 MWe class that is designed to be used in single stand-alone units or in small multi-unit plants. CANDU 6 units were built in Quebec and New Brunswick, as well as Pakistan, Argentina, South Korea, Romania, and China. A single example of a non-CANDU 6 design was sold to India. The multi-unit design was used o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium Compounds
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties. Gadolinium was discovered in 1880 by Jean Charles de Marignac, who detected its oxide by using spectroscopy. It is named after the mineral gadolinite, one of the minerals in which gadolinium is found, itself named for the Finnish chemist Johan Gadolin. Pure gadolinium was first isolated by the chemist Paul-Émile Lecoq de Boisbaudran around 1886. Gadoliniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrates
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures: Dietary nitrate A rich source of inorganic nitrate in the human diets come from leafy green foods ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |