|
GPX4
Glutathione peroxidase 4, also known as GPX4, is an enzyme that in humans is encoded by the ''GPX4'' gene. GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation. Function The antioxidant enzyme glutathione peroxidase 4 (GPX4) belongs to the family of glutathione peroxidases, which consists of 8 known mammalian isoenzymes (GPX1–8). GPX4 catalyzes the reduction of hydrogen peroxide, organic hydroperoxides, and lipid peroxides at the expense of reduced glutathione and functions in the protection of cells against oxidative stress. The oxidized form of glutathione (glutathione disulfide), which is generated during the reduction of hydroperoxides by GPX4, is recycled by glutathione reductase and NADPH/H+. GPX4 differs from the other GPX family members in terms of its monomeric structure, a less restricted dependence on glutathione as reducing substrate, and the ability to reduce lipid-hydroperoxides inside biological membranes. Inactiva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferroptosis
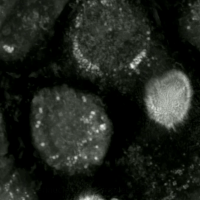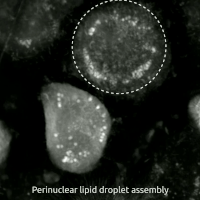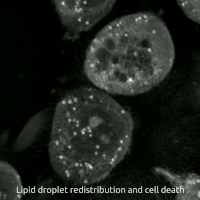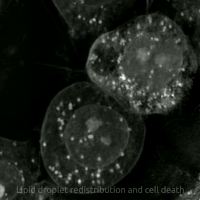
Ferroptosis is a type of programmed cell death dependent on iron and characterized by the accumulation of lipid peroxides, and is genetically and biochemically distinct from other forms of regulated cell death such as apoptosis. Ferroptosis is initiated by the failure of the glutathione-dependent antioxidant defenses, resulting in unchecked lipid peroxidation and eventual cell death. Lipophilic antioxidants and iron chelators can prevent ferroptotic cell death. Although the connection between iron and lipid peroxidation has been appreciated for years, it was not until 2012 that Brent Stockwell and Scott J. Dixon coined the term ferroptosis and described several of its key features. Researchers have identified roles in which ferroptosis can contribute to the medical field, such as the development of cancer therapies. Ferroptosis activation plays a regulatory role on growth of tumor cells in the human body. However, the positive effects of ferroptosis could be potentially neutralized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione Peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water. Isozymes Several isozymes are encoded by different genes, which vary in cellular location and substrate specificity. Glutathione peroxidase 1 (GPx1) is the most abundant version, found in the cytoplasm of nearly all mammalian tissues, whose preferred substrate is hydrogen peroxide. Glutathione peroxidase 4 (GPx4) has a high preference for lipid hydroperoxides; it is expressed in nearly every mammalian cell, though at much lower levels. Glutathione peroxidase 2 is an intestinal and extracellular enzyme, while glutathione peroxidase 3 is extracellular, especially abundant in plasma. So far, eight different isoforms of glutathione peroxidase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenoprotein
In molecular biology a selenoprotein is any protein that includes a selenocysteine (Sec, U, Se-Cys) amino acid residue. Among functionally characterized selenoproteins are five glutathione peroxidases (GPX) and three thioredoxin reductases, (TrxR/TXNRD) which both contain only one Sec. Selenoprotein P is the most common selenoprotein found in the plasma. It is unusual because in humans it contains 10 Sec residues, which are split into two domains, a longer N-terminal domain that contains 1 Sec, and a shorter C-terminal domain that contains 9 Sec. The longer N-terminal domain is likely an enzymatic domain, and the shorter C-terminal domain is likely a means of safely transporting the very reactive selenium atom throughout the body. Species distribution Selenoproteins exist in all major domains of life, eukaryotes, bacteria and archaea. Among eukaryotes, selenoproteins appear to be common in animals, but rare or absent in other phyla -one has been identified in the green alga ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPX2 (gene)
Glutathione peroxidase 2 is an enzyme that in humans is encoded by the ''GPX2'' gene. This gene is a member of the glutathione peroxidase family encoding a selenium-dependent glutathione peroxidase that is one of two isoenzymes responsible for the majority of the glutathione-dependent hydrogen peroxide-reducing activity in the epithelium of the gastrointestinal tract. Studies in knockout mice indicate that mRNA expression levels respond to luminal microflora, suggesting a role of the ileal glutathione peroxidases in preventing inflammation in the GI tract. The antioxidant enzyme glutathione peroxidase 2 (Gpx2) is one out of eight known glutathione peroxidases (Gpx1-8) in humans. Mammalian Gpx1, GPx2 (this protein), Gpx3, and Gpx4 have been shown to be selenium-containing enzymes, whereas Gpx6 is a selenoprotein in humans with cysteine-containing homologues in rodents. In selenoproteins, the 21st amino acid selenocysteine Selenocysteine (symbol Sec or U, in older publications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipid-hydroperoxide Glutathione Peroxidase
In enzymology, a phospholipid-hydroperoxide glutathione peroxidase () is an enzyme that catalyzes the chemical reaction :2 glutathione + a lipid hydroperoxide \rightleftharpoons glutathione disulfide + lipid + 2 H2O Thus, the two substrates of this enzyme are glutathione and lipid hydroperoxide, whereas its 3 products are glutathione disulfide, lipid, and H2O. This enzyme belongs to the family of oxidoreductases, to be specific those acting on a peroxide as acceptor (peroxidases). The systematic name of this enzyme class is glutathione:lipid-hydroperoxide oxidoreductase. Other names in common use include peroxidation-inhibiting protein, PHGPX, peroxidation-inhibiting protein: peroxidase, glutathione, (phospholipid hydroperoxide-reducing), phospholipid hydroperoxide glutathione peroxidase, hydroperoxide glutathione peroxidase, or glutathione peroxidase 4 (GPX4). This enzyme participates in glutathione metabolism Glutathione (GSH, ) is an antioxidant in plants, animals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Peroxidation
Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs). Initiation Initiation is the step in which a fatty acid radical is produced. The most notable initiators in living cells are reactive oxygen species (ROS), such as OH· and HOO·, which combines with a hydrogen atom to make water and a fatty acid radical. Propagation The fatty acid radical is not a very sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricants, to prevent oxidation, and to foods to prevent spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress. The only dietary antioxidants are vitamins A, C, and E, but the term ''antioxidant'' has also been applied to numerous other dietary compounds that only have antioxidant properties in vitro, with little evidence for antioxidant properties in vivo. Dietary supplements marketed as antioxidants have not been shown to maintain health or prevent disease in humans. History As part of their adaptation from marine life, terrestrial plants began producing non-marine anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPX1
Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the ''GPX1'' gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans. Structure This gene encodes a member of the glutathione peroxidase family, consisting of eight known glutathione peroxidases (GPx1-8) in humans. Mammalian Gpx1 (this gene), Gpx2, Gpx3, and Gpx4 have been shown to be selenium-containing enzymes, whereas Gpx6 is a selenoprotein in humans with cysteine-containing homologues in rodents. In selenoproteins, the 21st amino acid selenocysteine is inserted in the nascent polypeptide chain during the process of translational recoding of the UGA stop codon. In addition to the UGA-codon, a cis-acting element in the mRNA, called SECIS, binds SBP2 to recruit other proteins, such as eukaryotic elongation factor seleno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stop Codon
In molecular biology (specifically protein biosynthesis), a stop codon (or termination codon) is a codon ( nucleotide triplet within messenger RNA) that signals the termination of the translation process of the current protein. Most codons in messenger RNA correspond to the addition of an amino acid to a growing polypeptide chain, which may ultimately become a protein; stop codons signal the termination of this process by binding release factors, which cause the ribosomal subunits to disassociate, releasing the amino acid chain. While start codons need nearby sequences or initiation factors to start translation, a stop codon alone is sufficient to initiate termination. Properties Standard codons In the standard genetic code, there are three different termination codons: Alternative stop codons There are variations on the standard genetic code, and alternative stop codons have been found in the mitochondrial genomes of vertebrates, '' Scenedesmus obliquus'', and ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant. Structure and chemistry "Methylmercury" is a shorthand for the hypothetical "methylmercury cation", sometimes written "''methylmercury(1+) cation''" or "''methylmercury(II) cation''". This functional group is composed of a methyl group bonded to an atom of mercury. Its chemical formula is (sometimes written as ).The Methylmercury compound has an overall charge of +1, with Hg in the +2 oxidation state.Methylmercury exists as a substituent in many complexes of the type (L = Lewis base) and MeHgX (X = anion). As a positively charged ion it readily combines with anions such as chloride (), hydroxide () and nitrate (). It has particular affinity for sulfur-containing anions, particularly thiol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knockout Mouse
A knockout mouse, or knock-out mouse, is a genetically modified mouse (''Mus musculus'') in which researchers have inactivated, or " knocked out", an existing gene by replacing it or disrupting it with an artificial piece of DNA. They are important animal models for studying the role of genes which have been sequenced but whose functions have not been determined. By causing a specific gene to be inactive in the mouse, and observing any differences from normal behaviour or physiology, researchers can infer its probable function. Mice are currently the laboratory animal species most closely related to humans for which the knockout technique can easily be applied. They are widely used in knockout experiments, especially those investigating genetic questions that relate to human physiology. Gene knockout in rats is much harder and has only been possible since 2003. The first recorded knockout mouse was created by Mario R. Capecchi, Martin Evans, and Oliver Smithies in 1989, for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |