|
Fukuyama Reduction
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama. In the original scope of the reaction the silyl hydride was triethylsilane and the catalyst palladium on carbon: : Fukuyama reductions are used for the conversion of carboxylic acids (as thioester precursor) to aldehydes which is considered a difficult procedure because of the ease of secondary reduction to an alcohol. Reaction mechanism The basic reaction mechanism In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ... for this reaction takes place as a catalytic cycle: * Oxidative addition: *: + Pd^0 -> RC(O)-Pd^-SR * Transmetallation: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tohru Fukuyama
is a Japanese organic chemist and Professor of Chemistry at University of Tokyo in Japan. He discovered the Fukuyama coupling in 1998. Biography Fukuyama studied chemistry at Nagoya University with degrees Bachelor's (1971) and Master's (1973) degrees. As a graduate student, he then worked at Harvard University, where he received his doctorate in 1977 as an academic student of Yoshito Kishi. Until 1978, he continued his research as a postdoc in the Department of Chemistry of Harvard University and then moved to Rice University as an assistant professor, where in 1988 he obtained the rank of a chair holder. In 1995, he accepted a professorship in Pharmaceutical Sciences from the University of Tokyo, Japan. Since 2013, Fukuyama has been working as a professor at the Nagoya University - more precisely: Designated Professor of Pharmaceutical Sciences. The 2015 Nobel Prize in Physiology or Medicine winner Satoshi Ōmura is his old friend. Achievements * Fukuyama reduction *Fukuyama ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials science, etc. Since catalysts are regenerated, catalytic cycles are usually written as a sequence of chemical reactions in the form of a loop. In such loops, the initial step entails binding of one or more reactants by the catalyst, and the final step is the release of the product and regeneration of the catalyst. Articles on the Monsanto process, the Wacker process, and the Heck reaction show catalytic cycles. A catalytic cycle is not necessarily a full reaction mechanism. For example, it may be that the intermediates have been detected, but it is not known by which mechanisms the actual elementary reactions occur. Precatalysts Precatalysts are not catalysts but are ''precursors'' to catalysts. Precatalysts are converted in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Nucleophile
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form). Formally, a carbanion is the conjugate base of a carbon acid: :R3CH\, + \ddot^- -> \mathbf + HB where B stands for the base. The carbanions formed from deprotonation of alkanes (at an sp3 carbon), alkenes (at an sp2 carbon), arenes (at an sp2 carbon), and alkynes (at an sp carbon) are known as alkyl, alkenyl (vinyl), aryl, and alkynyl (acetylide) anions, respectively. Carbanions have a concentration of electron density at the negatively charged carbon, which, in most cases, reacts efficiently with a variety of electrophiles of varying strengths, including carbonyl groups, imines/ iminium salts, halogenating reagents (e.g., ''N''-bromosuccinimide and diiodine), and proton donors. A carbanion is one of several reactive intermediates in organic chemistry. In organic synthesis, organolithium reagents and G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fukuyama Coupling
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998. Advantages are high chemoselectivity, mild reaction conditions and the use of less-toxic reagents. : One advantage of this method is that the reaction stops at the ketone and does not proceed to a tertiary alcohol. In addition, the protocol is compatible with functional groups such as ketones, acetates, sulfides, aromatic bromides, chlorides and aldehydes. : The reaction (interrupted) has been used in the synthesis of biotin Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', bor ... : This reaction was preceded by the conceptually related Fuku ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BODIPY Synthesis Arroyo 2009
BODIPY is the technical common name of a chemical compound with formula , whose molecule consists of a boron difluoride group joined to a dipyrromethene group ; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-''s''-indacene in the IUPAC nomenclature. The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol. The compound itself was isolated only in 2009, but many derivatives—formally obtained by replacing one or more hydrogen atoms by other functional groups—have been known since 1968, and comprise the important class of BODIPY dyes.Alfred Treibs und Franz-Heinrich Kreuzer. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Annalen der Chemie 1968, 718 (1): 208-223. These organoboron compounds have attracted much interest as fluorescent dyes and markers in biological research. Structure In its crystalline solid form, the core BODIPY is almost, but not ent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
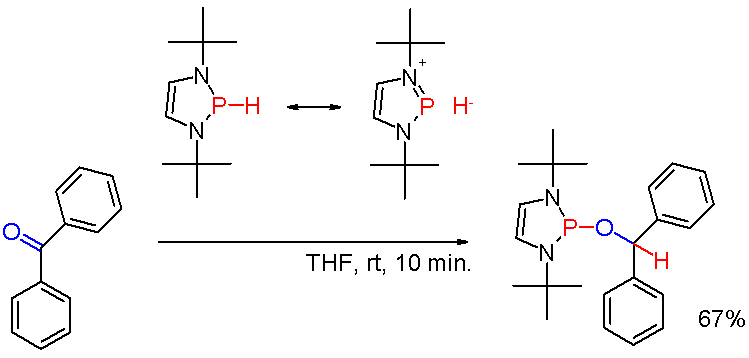
Organophosphine
Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphines (''n'' = 2), tertiary phosphines (''n'' = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3). Annette Schier and Hubert Schmidbaur"P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim. 1° vs 2° vs 3° phosphines Organophophines are classified according to the number of organic substituents. Primary phosphines Primary (1°) phosphines, with the formula RPH2, are typically prepared by alkylation of phosphine. Simple alkyl derivatives such as methylphosphine (CH3PH2) are prepared by alkylation of alkali metal derivatives MPH2 (M is Li, Na, or K). Another synthetic route in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pd(dba)2
Tris(dibenzylideneacetone)dipalladium(0) or d2(dba)3is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Because the dba ligands are easily displaced, the complex is used as a homogeneous catalyst in organic synthesis. Preparation and structure First reported in 1970, it is prepared from dibenzylideneacetone and sodium tetrachloropalladate. Because it is commonly recrystallized from chloroform, the complex is often supplied as the adduct d2(dba)3·CHCl3Jiro Tsuji and Ian J. S. Fairlamb "Tris(dibenzylideneacetone)dipalladium–Chloroform" E-EROS, 2008. The purity of samples can be variable. In d2(dba)3 the pair of Pd atoms are separated by 320 pm but are tied together by dba units. The Pd(0) centres are bound to the alkene parts of the dba ligands. Applications d2(dba)3is used as a source of soluble Pd(0), in particular as a catalys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I)-thiophene-2-carboxylate
Copper(I) thiophene-2-carboxylate or CuTC is a coordination complex derived from copper and thiophene-2-carboxylic acid. It is used as a reagent to promote the Ullmann reaction between aryl halide In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...s. : References {{reflist Thiophenes Copper(I) compounds Reagents for organic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BODIPY
BODIPY is the technical common name of a chemical compound with formula , whose molecule consists of a boron difluoride group joined to a dipyrromethene group ; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-''s''-indacene in the IUPAC nomenclature. The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol. The compound itself was isolated only in 2009, but many derivatives—formally obtained by replacing one or more hydrogen atoms by other functional groups—have been known since 1968, and comprise the important class of BODIPY dyes.Alfred Treibs und Franz-Heinrich Kreuzer. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Annalen der Chemie 1968, 718 (1): 208-223. These organoboron compounds have attracted much interest as fluorescent dyes and markers in biological research. Structure In its crystalline solid form, the core BODIPY is almost, but not entire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is often the product-forming step in many catalytic processes. Since oxidative addition and reductive elimination are reverse reactions, the same mechanisms apply for both processes, and the product equilibrium depends on the thermodynamics of both directions. General information Reductive elimination is often seen in higher oxidation states, and can involve a two-electron change at a single metal center (mononuclear) or a one-electron change at each of two metal centers (binuclear, dinuclear, or bimetallic). For mononuclear reductive elimination, the oxidation state of the metal decreases by two, while the d-electron count of the metal increases by two. This pathway is common for d8 metals Ni(II), Pd(II), and Au(III) and d6 metals P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmetallation
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form: :M1–R + M2–R′ → M1–R′ + M2–R where R and R′ can be, but are not limited to, an alkyl, aryl, alkynyl, allyl, halogen, or pseudohalogen group. The reaction is usually an irreversible process due to thermodynamic and kinetic reasons. Thermodynamics will favor the reaction based on the electronegativities of the metals and kinetics will favor the reaction if there are empty orbitals on both metals. There are different types of transmetalation including redox-transmetalation and redox-transmetalation/ligand exchange. During transmetalation the metal-carbon bond is activated, leading to the formation of new metal-carbon bonds. Transmetalation is commonly used in catalysis, synthesis of main group complexes, and synthesis of transition metal complexes. Types of transmetalation There are two main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


dipalladium(0).jpg)


