|
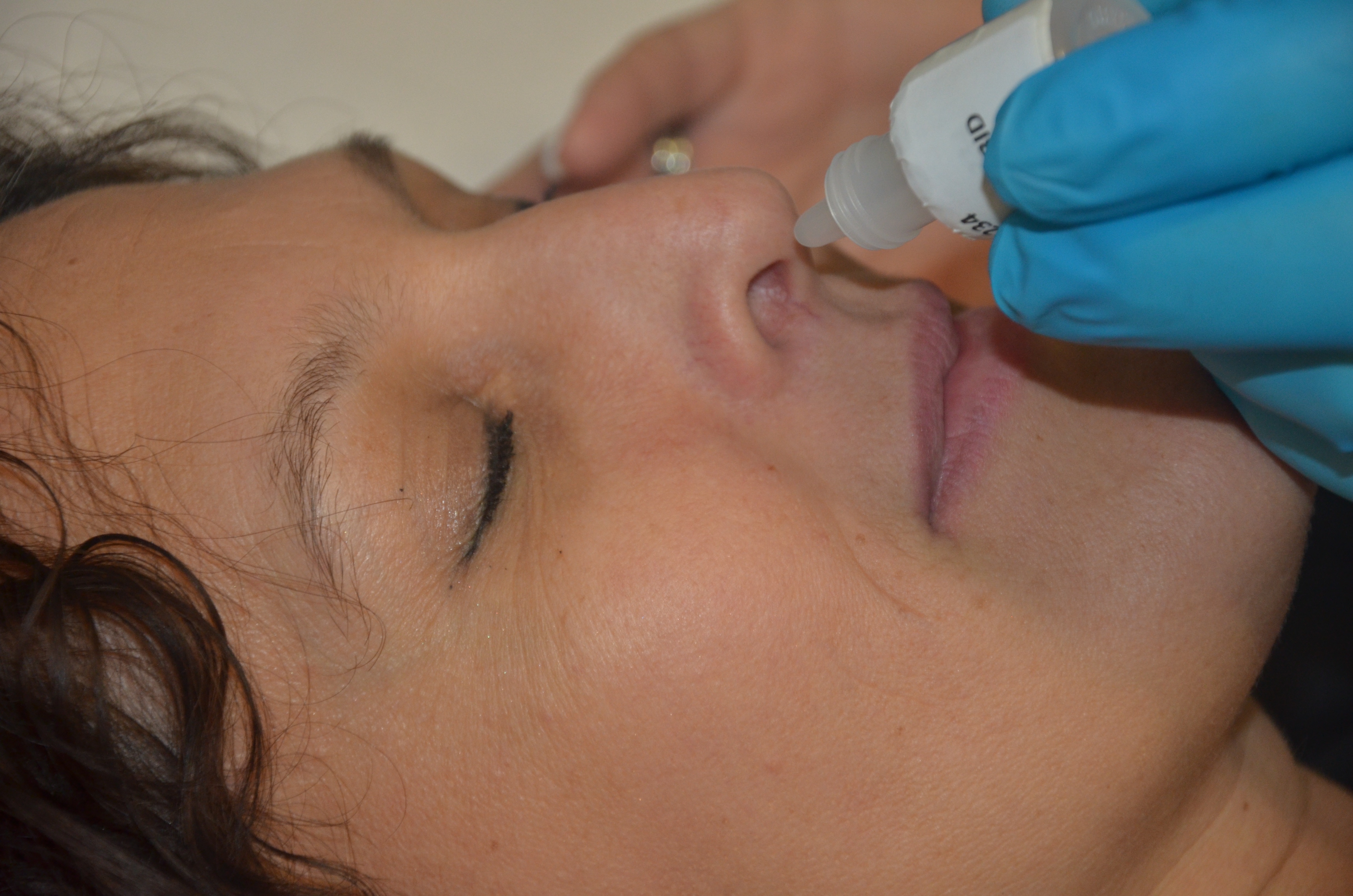
Fluticasone Furoate
Fluticasone furoate, sold under the brand name Flonase Sensimist and formerly Veramyst, among others, is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray. It is also available as an inhaled corticosteroid to help prevent and control symptoms of asthma. It is derived from cortisol. Unlike fluticasone propionate, which is only approved for children four years and older, fluticasone furoate is approved in children as young as two years of age when used for allergies. It was approved for medical use in the United States in April 2007, and in the European Union in November 2008. In 2020, fluticasone was the 23rd most commonly prescribed medication in the United States, with more than 24million prescriptions. Society and culture Brand names In the US it is marketed by GlaxoSmithKline for asthma as Arnuity Ellipta and is only available with a prescription. It is marketed over-the-counter for allergic rhinitis as Flonase Sen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nasal Administration
Nasal administration, popularly known as snorting, is a route of administration in which drugs are insufflated through the nose. It can be a form of either topical administration or systemic administration, as the drugs thus locally delivered can go on to have either purely local or systemic effects. Nasal sprays are locally acting drugs such as decongestants for cold and allergy treatment, whose systemic effects are usually minimal. Examples of systemically active drugs available as nasal sprays are migraine drugs, rescue medications for overdose and seizure emergencies, nicotine replacement, and hormone treatments. Advantages with nasal systemic drug delivery The nasal cavity is covered by a thin mucosa which is well vascularised. Therefore, a drug molecule can be transferred quickly across the single epithelial cell layer directly to the systemic blood circulation without first-pass hepatic and intestinal metabolism. The effect is often reached within 5 min for smaller ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GlaxoSmithKline
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucocorticoids
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau (glucose + cortex + steroid) and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure (see structure below). Glucocorticoids are part of the feedback mechanism in the immune system, which reduces certain aspects of immune function, such as inflammation. They are therefore used in medicine to treat diseases caused by an overactive immune system, such as allergies, asthma, autoimmune diseases, and sepsis. Glucocorticoids have many diverse (pleiotropic) effects, including potentially harmful side effects. They also interfere with some of the abnormal mechanisms in cancer cells, so they are used in high doses to tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GSK Plc Brands , a rendering pipeline
{{disambiguation ...
GSK may refer to: * Galatasaray S.K., a Turkish sports club based in Istanbul * Glycogen synthase kinase * Golden State Killer, a California serial rapist and murderer * GSK plc, formerly GlaxoSmithKline, a multinational pharmaceutical corporation * GTK Scene Graph Kit GTK Scene Graph Kit (GSK) is the rendering and scene graph API for GTK introduced with version 3.90. GSK lies between the graphical control elements (widgets) and the rendering. Like GDK, GSK is part of GTK and licensed under the GNU Lesser Ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corticosteroid Esters
This is a list of corticosteroid esters, including esters of steroidal glucocorticoids and mineralocorticoids. Esters of natural corticosteroids Desoxycortone esters * Desoxycortone acetate (deoxycortone acetate; desoxycorticosterone acetate) * Desoxycortone cypionate (deoxycortone cypionate; desoxycorticosterone cypionate) * Desoxycortone enanthate (deoxycortone enanthate; deoxycorticosterone enanthate) * Desoxycortone glucoside (deoxycortone glucoside; deoxycorticosterone glucoside) * Desoxycortone pivalate (deoxycortone pivalate; deoxycorticosterone pivalate) Hydrocortisone esters * Benzodrocortisone (hydrocortisone 17-benzoate) * Hydrocortamate (hydrocortisone 21-(diethylamino)acetate) * Hydrocortisone aceponate (hydrocortisone 21-acetate 17α-propionate) * Hydrocortisone acetate * Hydrocortisone bendazac * Hydrocortisone buteprate (hydrocortisone 17α-butyrate 21-propionate) * Hydrocortisone butyrate (hydrocortisone 17α-butyrate) * Hydrocortisone 21-butyrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluticasone Propionate/salmeterol
Fluticasone/salmeterol, sold under the brand name Advair among others, is a fixed-dose combination medication containing fluticasone propionate and salmeterol. It is used in the management of asthma and chronic obstructive pulmonary disease (COPD). It is used by inhaling the medication into the lungs. Common side effects include thrush, headache, and cough. Serious side effects may include worsening asthma, anaphylaxis, seizures, and heart problems. Safety in pregnancy and breastfeeding is unclear. Fluticasone, a corticosteroid, works by decreasing inflammation while salmeterol, a long-acting beta-adrenoceptor agonist (LABA), works by activating beta-2 adrenergic receptors. The combination was approved for medical use in the United States in 2000. A generic version was approved in the United States in 2019. In 2020, it was the 56th most commonly prescribed medication in the United States, with more than 11million prescriptions. Medical uses Fluticasone, a corticosteroid, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluticasone Furoate/umeclidinium Bromide/vilanterol
Fluticasone furoate/umeclidinium bromide/vilanterol, sold under the brand name Trelegy Ellipta among others, is a fixed-dose combination inhaled medication that is used for the maintenance treatment of chronic obstructive pulmonary disease (COPD). The medications work in different ways: fluticasone furoate is an inhaled corticosteroid (ICS), umeclidinium is a long-acting muscarinic antagonist (LAMA), and vilanterol is a long-acting beta-agonist (LABA). In 2020, it was the 224th most commonly prescribed medication in the United States, with more than 2million prescriptions. Medical uses The combination fluticasone furoate/umeclidinium bromide/vilanterol product is approved by the United States Food and Drug Administration with an indication for the maintenance treatment of a chronic lung problem called chronic obstructive pulmonary disease (COPD) in adults who (1) have already tried fluticasone furoate/vilanterol (brand name Breo Ellipta) but are still experiencing symptoms o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a type of progressive lung disease characterized by long-term respiratory symptoms and airflow limitation. The main symptoms include shortness of breath and a cough, which may or may not produce mucus. COPD progressively worsens, with everyday activities such as walking or dressing becoming difficult. While COPD is incurable, it is preventable and treatable. The two most common conditions of COPD are emphysema and chronic bronchitis and they have been the two classic COPD phenotypes. Emphysema is defined as enlarged airspaces ( alveoli) whose walls have broken down resulting in permanent damage to the lung tissue. Chronic bronchitis is defined as a productive cough that is present for at least three months each year for two years. Both of these conditions can exist without airflow limitation when they are not classed as COPD. Emphysema is just one of the structural abnormalities that can limit airflow and can exist without ai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluticasone Furoate/vilanterol
Fluticasone furoate/vilanterol (FF/VI), sold under the brand name Breo Ellipta among others, is a combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains fluticasone furoate, an inhaled corticosteroid, and vilanterol, an ultra- long-acting β2 agonist (ultra-LABA). In 2013, the drug was approved for use in the United States by the Food and Drug Administration (FDA) for long-term maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and emphysema, and the European Medicines Agency approved it as a second-line therapy for the treatment of COPD and asthma. There were, however, concerns that LABAs such as vilanterol increase the risk of deaths due to asthma. In 2017, the FDA states that they were not justified. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 115th most commonly prescribed medication in the United States, with more than 5mill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combination Drug
A combination drug or a fixed-dose combination (FDC) is a medicine that includes two or more active ingredients combined in a single dosage form. Terms like "combination drug" or "combination drug product" can be common shorthand for a FDC product (since most combination drug products are currently FDCs), although the latter is more precise if in fact referring to a mass-produced product having a predetermined combination of drugs and respective dosages (as opposed to ''customized'' polypharmacy via compounding). And it should also be distinguished from the term "combination product" in medical contexts, which without further specification can refer to products that combine different ''types'' of medical products—such as device/drug combinations as opposed to drug/drug combinations. Note that when a combination drug product (whether fixed-dose or not) is a "pill" (i.e., a tablet or capsule), then it may also be a kind of "polypill" or combopill. Initially, fixed-dose combinati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |