|
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
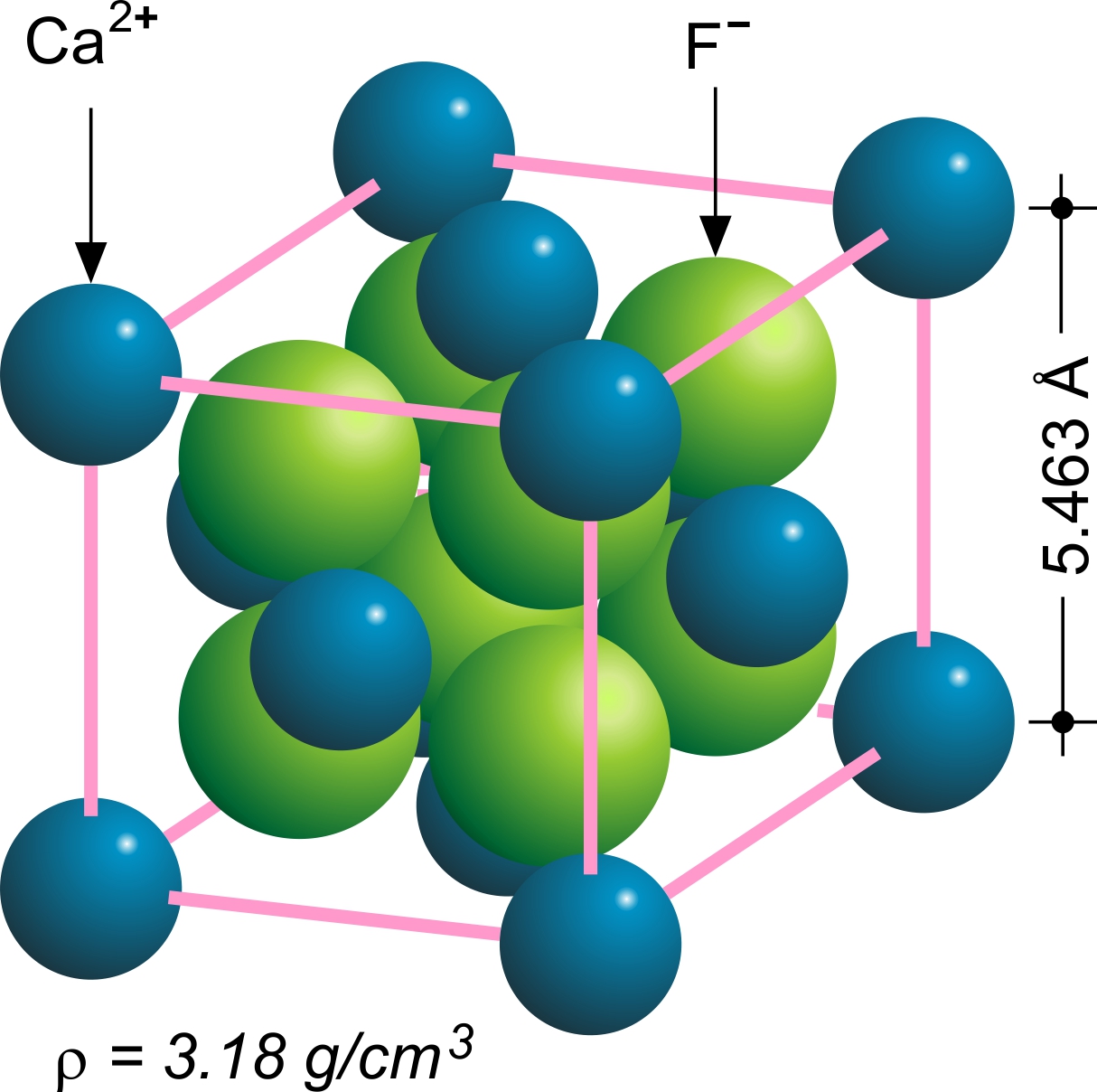
Calcium Fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities. Chemical structure The compound crystallizes in a cubic motif called the fluorite structure. Ca2+ centres are eight-coordinate, being centered in a cube of eight F− centres. Each F− centre is coordinated to four Ca2+ centres in the shape of a tetrahedron. Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers. The same crystal structure is found in numerous ionic compounds with formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding anti-structure, called the antifluorite structure, anions and cations are swapped, such as Be2C. Gas phase The gas phase is noteworthy for failing the predictions of VSEPR theory; the molecule is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide Mineral
Halide minerals are those minerals with a dominant halide anion (, , and ). Complex halide minerals may also have polyatomic anions. Examples include the following: *Atacamite * Avogadrite (K,Cs)BF *Bararite (β) *Bischofite * Brüggenite *Calomel *Carnallite *Carnallite * Cerargyrite/Horn silver AgCl * Chlorargyrite AgCl, bromargyrite AgBr, and iodargyrite AgI *Cryolite *Cryptohalite (a) Vanadates), 09 Silicates: * ''neso-'': insular (from Greek , "island") * ''soro-'': grouped (from Greek , "heap, pile, mound") * ''cyclo-'': ringed (from Greek , "circle") * ''ino-'': chained (from Greek , "fibre", rom Ancient Greek * ''phyllo-'': sheeted (from Greek , "leaf") * ''tecto-'': of three-dimensional framework (from Greek , "of building") ;Nickel–Strunz code scheme ''NN.XY.##x'': * ''NN'': Nickel–Strunz mineral class number * ''X'': Nickel–Strunz mineral division letter * ''Y'': Nickel–Strunz mineral family letter * ''##x'': Nickel–Strunz mineral/group n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide Mineral
Halide minerals are those minerals with a dominant halide anion (, , and ). Complex halide minerals may also have polyatomic anions. Examples include the following: *Atacamite * Avogadrite (K,Cs)BF *Bararite (β) *Bischofite * Brüggenite *Calomel *Carnallite *Carnallite * Cerargyrite/Horn silver AgCl * Chlorargyrite AgCl, bromargyrite AgBr, and iodargyrite AgI *Cryolite *Cryptohalite (a) Vanadates), 09 Silicates: * ''neso-'': insular (from Greek , "island") * ''soro-'': grouped (from Greek , "heap, pile, mound") * ''cyclo-'': ringed (from Greek , "circle") * ''ino-'': chained (from Greek , "fibre", rom Ancient Greek * ''phyllo-'': sheeted (from Greek , "leaf") * ''tecto-'': of three-dimensional framework (from Greek , "of building") ;Nickel–Strunz code scheme ''NN.XY.##x'': * ''NN'': Nickel–Strunz mineral class number * ''X'': Nickel–Strunz mineral division letter * ''Y'': Nickel–Strunz mineral family letter * ''##x'': Nickel–Strunz mineral/group n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromatic Aberration
In optics, chromatic aberration (CA), also called chromatic distortion and spherochromatism, is a failure of a lens to focus all colors to the same point. It is caused by dispersion: the refractive index of the lens elements varies with the wavelength of light. The refractive index of most transparent materials decreases with increasing wavelength. Since the focal length of a lens depends on the refractive index, this variation in refractive index affects focusing. Chromatic aberration manifests itself as "fringes" of color along boundaries that separate dark and bright parts of the image. Types There are two types of chromatic aberration: ''axial'' (''longitudinal''), and ''transverse'' (''lateral''). Axial aberration occurs when different wavelengths of light are focused at different distances from the lens (focus ''shift''). Longitudinal aberration is typical at long focal lengths. Transverse aberration occurs when different wavelengths are focused at different positions i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material polytetrafluoroethylene, PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to Etching (microfabrication), etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Polytetrafluoroethylene, Teflon, fluoropolymers, fluorocarbons, and refrigeration, refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flux (metallurgy)
In metallurgy, a flux () is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining. Some of the earliest known fluxes were sodium carbonate, potash, charcoal, coke, borax, lime, lead sulfide and certain minerals containing phosphorus. Iron ore was also used as a flux in the smelting of copper. These agents served various functions, the simplest being a reducing agent, which prevented oxides from forming on the surface of the molten metal, while others absorbed impurities into the slag, which could be scraped off the molten metal. Fluxes are also used in foundries for removing impurities from molten nonferrous metals such as aluminium, or for adding desirable trace elements such as titanium. As cleaning agents, fluxes facilitate soldering, brazing, and welding by removing oxidation from the metals to be joined. In some applications molten flux also serve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Habit
In mineralogy, crystal habit is the characteristic external shape of an individual crystal or crystal group. The habit of a crystal is dependent on its crystallographic form and growth conditions, which generally creates irregularities due to limited space in the crystallizing medium (commonly in rocks).Klein, Cornelis, 2007, ''Minerals and Rocks: Exercises in Crystal and Mineral Chemistry, Crystallography, X-ray Powder Diffraction, Mineral and Rock Identification, and Ore Mineralogy,'' Wiley, third edition, Wenk, Hans-Rudolph and Andrei Bulakh, 2004, ''Minerals: Their Constitution and Origin,'' Cambridge, first edition, Recognizing the habit can aid in mineral identification and description, as the crystal habit is an external representation of the internal ordered atomic arrangement. Most natural crystals, however, do not display ideal habits and are commonly malformed. Hence, it is also important to describe the quality of the shape of a mineral specimen: * Euhedral: a cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohs Scale Of Mineral Hardness
The Mohs scale of mineral hardness () is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of various minerals through the ability of harder material to scratch softer material. The scale was introduced in 1812 by the German geologist and mineralogist Friedrich Mohs, in his book ''"Versuch einer Elementar-Methode zur naturhistorischen Bestimmung und Erkennung der Fossilien"''; it is one of several definitions of hardness in materials science, some of which are more quantitative. The method of comparing hardness by observing which minerals can scratch others is of great antiquity, having been mentioned by Theophrastus in his treatise ''On Stones'', , followed by Pliny the Elder in his ''Naturalis Historia'', . The Mohs scale is useful for identification of minerals in the field, but is not an accurate predictor of how well materials endure in an industrial setting – ''toughness''. Minerals The Mohs scale of mineral hardness is based on the ability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoluminescence
Thermoluminescence is a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon heating of the material. The phenomenon is distinct from that of black-body radiation. Physics High energy radiation creates electronic excited states in crystalline materials. In some materials, these states are ''trapped'', or ''arrested'', for extended periods of time by localized defects, or imperfections, in the lattice interrupting the normal intermolecular or inter-atomic interactions in the crystal lattice. Quantum-mechanically, these states are stationary states which have no formal time dependence; however, they are not stable energetically, as vacuum fluctuations are always "prodding" these states. Heating the material enables the trapped states to interact with phonons, i.e. lattice vibrations, to rapidly decay into lower-ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)
_0468.jpg)



