|
Flexplay
Flexplay is a trademark for a discontinued DVD-compatible optical video disc format with a time-limited (usually 48-hour) playback. They are often described as "self-destructing", although the disc merely turns black or dark red and does not physically disintegrate. The technology launched in August 2003 as a joint-venture with Disney's Buena Vista Home Entertainment under the name eZ-D. The Flexplay concept was invented by two professors, Yannis Bakos and Erik Brynjolfsson, who founded Flexplay Technologies in 1999. The technology was developed by Flexplay Technologies and General Electric. Origins The technology was originally intended as an alternative means for the short-term rental of newly released movies. Since the disc is capable of being used in any standard DVD player, the manufacturers hoped that it would succeed where other time-limited DVD technologies, such as DIVX, failed. Test marketing of eZ-D discs began in August 2003, but was canceled early when consumers rej ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yannis Bakos
Yannis Bakos is a professor at the Leonard N. Stern School of Business at New York University. His primary area of expertise is the economic and business implications of information technology, the Internet, and online media. He is the co-founder (with Chris F. Kemerer) of thWorkshop on Information Systems and Economics(WISE), and the co-inventor of Flexplay DVDs. Early life Bakos holds a Ph.D. in Management and an MBA in Finance from the MIT Sloan School of Management. He also received a master's degree in Electrical Engineering and Computer Science and a B.S. in Computer Engineering from MIT's Department of Electrical Engineering and Computer Science. Before coming to NYU, Professor Bakos was on the faculty of thMerage School of Business at the University of California, Irvineand the Sloan School of Management at MIT. Career Bakos' early work showed that the internet would reduce the search costs of buyers and sellers, and that the resulting electronic marketplaces would result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
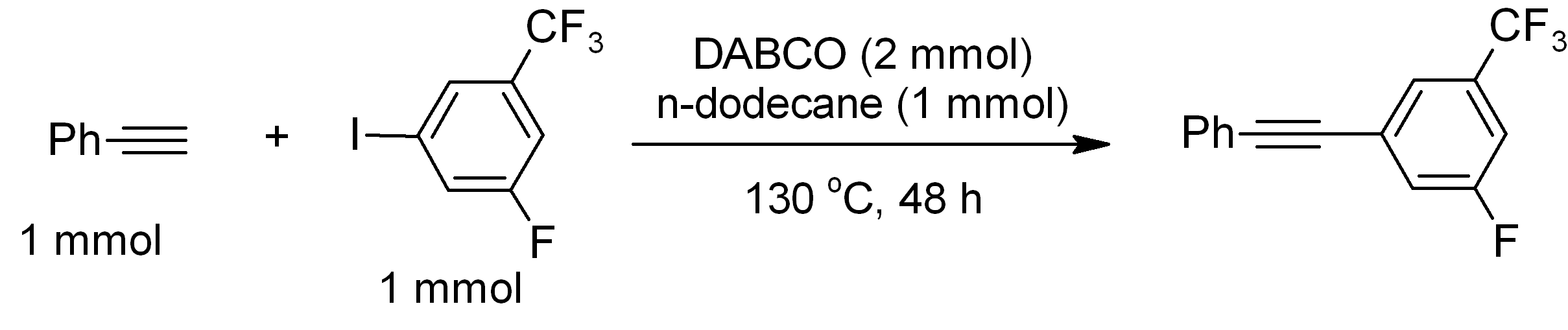
DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : Lewis ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leuco Dye
A leuco dye (from the Greek λευκός ''leukos'': white ) is a dye which can switch between two chemical forms; one of which is colorless. Reversible transformations can be caused by heat, light or pH; resulting in examples of thermochromism, photochromism and halochromism respectively. Irreversible transformations typically involve reduction or oxidation.Chemistry and Applications of Leuco Dyes. Ramaiah Muthyala. 302 pag. Springer; 1997 edition. The colorless form is sometimes referred to as the leuco form. Leuco dyes form the basis of thermal printer papers and certain pH indicators. Examples The most common example is in applying sulfur dyes and vat dyes; with indigo being a classic case. This is characteristically purple but is also completely insoluble in water, meaning that it cannot be applied to clothes directly. It is instead reduced to indigo white (sometimes Leucoindigo), which is water-soluble but colorless. When a submerged fabric is removed from a dyebath of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Disc
In computing and optical disc recording technologies, an optical disc (OD) is a flat, usually circular disc that encodes binary data (bits) in the form of pits and lands on a special material, often aluminum, on one of its flat surfaces. Its main uses are physical offline data distribution and long-term archival. Changes from pit to land or from land to pit correspond to a binary value of 1; while no change, regardless of whether in a land or a pit area, corresponds to a binary value of 0. Non-circular optical discs exist for fashion purposes; see shaped compact disc. Design and technology The encoding material sits atop a thicker substrate (usually polycarbonate) that makes up the bulk of the disc and forms a dust defocusing layer. The encoding pattern follows a continuous, spiral path covering the entire disc surface and extending from the innermost track to the outermost track. The data are stored on the disc with a laser or stamping machine, and can be accesse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brilliant Cresyl Blue
Brilliant cresyl blue is a supravital stain used for counting reticulocytes. It is classified as an oxazine dye Oxazines are heterocyclic compounds containing one oxygen and one nitrogen atom in a doubly unsaturated six-membered ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds. By extensi .... N95 dust masks, eye shields, and gloves must all be worn when handling the chemical. References {{heterocyclic-stub Chlorides Zinc compounds Oxazine dyes Phenoxazines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphorsulfonic Acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances. This compound is commercially available. It can be prepared by sulfonation of camphor with sulfuric acid and acetic anhydride: : Although this reaction appears to be a sulfonation of an unactivated methyl group, the actual mechanism is believed to involve a retro-semipinacol rearrangement, deprotonation next to the tertiary carbocation to form an alkene, sulfonation of the alkene intermediate, and finally, semipinacol rearrangement to re-establish the ketone function. In organic synthesis, CSA and its derivatives can be used as resolving agents for chiral amines and other cations. The synthesis of osanetant was an example of this. 3-bromocamphor-8-sulfonic acid was used in the synthesis of enantiopure devazepide. Camphorsu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water (molecule)
Water () is a Chemical polarity, polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from Color of water, an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a ice, solid, liquid, and water vapor, gas on Earth's surface. It is also the third most abundant molecule in the universe (behind Hydrogen, molecular hydrogen and carbon monoxide). Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Dithionite
Sodium dithionite (also known as sodium hydrosulfite) is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions. Structure The structure has been examined by Raman spectroscopy and single-crystal X-ray diffraction. The dithionite dianion has C symmetry, with almost eclipsed with a 16° O-S-S-O torsional angle. In the dihydrated form (), the dithionite anion has gauche 56° O-S-S-O torsional angle. A weak S-S bond is indicated by the S-S distance of 239 pm, which is elongated by ca. 30 pm relative to a typical S-S bond. Because this bond is fragile, the dithionite anion dissociates in solution into the O2sup>− radicals, as has been confirmed by EPR spectroscopy. It is also observed that 35S undergoes rapid exchange between S2O42− and SO2 in neutral or acidic solution, consistent with the weak S-S bond in the anion. Preparation Sodium dithionite is produced industrially by reduction of sulfur d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron donor, donates electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |