|
Fenske Equation
The Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux (i.e., which means that no overhead product distillate is being withdrawn from the column). The equation was derived in 1932 by Merrell Fenske, a professor who served as the head of the chemical engineering department at the Pennsylvania State University from 1959 to 1969. When designing large-scale, continuous industrial distillation towers, it is very useful to first calculate the minimum number of theoretical plates required to obtain the desired overhead product composition. Common versions of the Fenske equation This is one of the many different but equivalent versions of the Fenske equation valid only for binary mixtures: [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Reflux
Total may refer to: Mathematics * Total, the summation of a set of numbers * Total order, a partial order without incomparable pairs * Total relation, which may also mean ** connected relation (a binary relation in which any two elements are comparable). * Total function, a partial function that is also a total relation Business * TotalEnergies, a French petroleum company * Total (cereal), a food brand by General Mills * Total, a brand of strained yogurt made by Fage * Total, a database management system marketed by Cincom Systems * Total Linhas Aéreas - a brazilian airline * Total, a line of dental products by Colgate Music and culture * Total (group), an American R&B girl group * '' Total: From Joy Division to New Order'', a compilation album * ''Total'' (Sebastian album) * ''Total'' (Total album) * ''Total'' (Teenage Bottlerocket album) * ''Total'' (Seigmen album) * ''Total'' (Wanessa album) * ''Total'' (Belinda Peregrín album) * ''Total 1'', an annual compilation al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Christian Brothers University
Christian Brothers University is a private Roman Catholic higher education institution in Memphis, Tennessee. It was founded in 1871 by the De La Salle Christian Brothers, a Catholic teaching order. History Christian Brothers University was founded on November 19, 1871, by members of the Institute of the Brothers of the Christian Schools, a Roman Catholic religious order founded by St. John Baptist de la Salle, the patron saint of teachers. At foundation the educational institution was named Christian Brothers College which was changed to Christian Brothers University when the school became a university in June 1990.CBU History. Christian Brothers University. Accessed October 1, 2007. Founding Brother Maurelian was appointed the first president. His three terms as president totalled 31 years. Olde ...
|
McCabe–Thiele Method
The McCabe–Thiele method is a chemical engineering technique for the analysis of binary distillation. It uses the fact that the composition at each theoretical tray (or equilibrium stage) is completely determined by the mole fraction of one of the two components and is based on the assumption of constant molar overflow, which requires that: * the molar heats of vaporization of the feed components are equal; * for every mole of liquid vaporized, a mole of vapor is condensed; and * heat effects such as heat of solution are negligible. The method was first published by Warren L. McCabe and Ernest Thiele in 1925, both of whom were working at the Massachusetts Institute of Technology (MIT) at the time. Construction and use A McCabe–Thiele diagram for the distillation of a binary feed is constructed using the vapor-liquid equilibrium (VLE) data for the lower-boiling component of the feed. On a planar graph, the horizontal (x) axis represents the mole fraction of the liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or Cracking (chemistry), cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In Chemical industry, industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuous Distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling (or evaporation) and condensation. The process produces at least two output fractions. These fractions include at least one '' volatile'' distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a ''bottoms'' (or ''residuum'') fraction, which is the least volatile residue that has not been separately captured as a condensed vapor. An alternative to continuous distillation is batch distillation, where the mixture is added to the unit at the start of the distillation, distillate fractions are taken out sequentially in time (one after another) during the distillation, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature according ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium Stage
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process. Applications The concept of theoretical plates and trays or equilibrium stages is used in the design of many different types of separation. Distillation columns The concept of theoretical plates in designing distillation processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalton's Law
Dalton's law (also called Dalton's law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This empirical law was observed by John Dalton in 1801 and published in 1802.J. Dalton (1802)"Essay IV. On the expansion of elastic fluids by heat,"''Memoirs of the Literary and Philosophical Society of Manchester'', vol. 5, pt. 2, pages 595–602; see page 600. Dalton's law is related to the ideal gas laws. Formula Mathematically, the pressure of a mixture of non-reactive gases can be defined as the summation: p_\text = \sum_^n p_i = p_1+p_2+p_3+\cdots+p_n where ''p''1, ''p''2, ..., ''pn'' represent the partial pressures of each component. p_ = p_\text x_i where ''xi'' is the mole fraction of the ''i''th component in the total mixture of ''n'' components . Volume-based concentration The relationship below provides a way to determine the volume-based concentration of any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
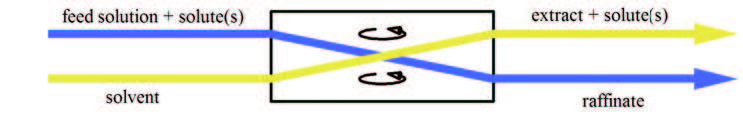
Liquid–liquid Extraction
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |