|
FL3 (flavagline)
FL3 is a synthetic flavagline that displays potent anticarcinogen, anticancer and cardioprotectant activities. This compound induces the death of cancer cells by an original mechanism that involves the apoptosis-inducing factor and caspase 12, suggesting that it may improve the efficacy of cancer chemotherapies. It was also shown that FL3 may enhance the efficacy of one of the most widely used anticarcinogen, anticancer agents, doxorubicin, and alleviate its main adverse effect, cardiac damage. References Diols Benzofuran ethers at the benzene ring Organobromides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavagline
Flavaglines are a family of natural products that are found in plants of the genus ''Aglaia'' (Meliaceae). These compounds are characterized by a cyclopenta[''b'']benzofuran skeleton. In 1982 King and colleagues discovered the first member of this family, rocaglamide, based on its antileukemic activity. Since then, about 50 other flavaglines have been characterized. These molecules display strong insecticidal, antifungal, anti-inflammatory, neuroprotective, cardioprotective and anticarcinogen, anticancer activities. In mouse models of cancer, flavaglines enhance the efficacy of chemotherapies and also alleviate the cardiac adverse effect of these chemotherapies. The challenge raised by their structural complexity has attracted the attention of some organic chemists. In 1990, Barry Trost presented an Enantiomer, enantioselective synthesis of rocaglamide in 18 steps and confirmed its absolute configuration. See also * FL3 (flavagline) *Rocaglamide *Silvestrol *Aglafoline Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticarcinogen
An anticarcinogen (also known as a carcinopreventive agent) is a substance that counteracts the effects of a carcinogen or inhibits the development of cancer. Anticarcinogens are different from anticarcinoma agents (also known as anticancer or anti-neoplastic agents) in that anticarcinoma agents are used to selectively destroy or inhibit cancer cells ''after'' cancer has developed. Interest in anticarcinogens is motivated primarily by the principle that it is preferable to prevent disease (preventive medicine) than to have to treat it ( rescue medicine). In theory, anticarcinogens may act via different mechanisms including enhancement of natural defences against cancer, deactivation of carcinogens, and blocking the mechanisms by which carcinogens act (such as free radical damage to DNA). Confirmation that a substance possesses anticarcinogenic activity requires extensive ''in vitro'', ''in vivo'', and clinical investigation. Health claims for anticarcinogens are regulated by v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis-inducing Factor
Apoptosis inducing factor is involved in initiating a caspase-independent pathway of apoptosis (positive intrinsic regulator of apoptosis) by causing DNA fragmentation and chromatin condensation. Apoptosis inducing factor is a flavoprotein. It also acts as an NADH oxidase. Another AIF function is to regulate the permeability of the mitochondrial membrane upon apoptosis. Normally it is found behind the outer membrane of the mitochondrion and is therefore secluded from the nucleus. However, when the mitochondrion is damaged, it moves to the cytosol and to the nucleus. Inactivation of AIF leads to resistance of embryonic stem cells to death following the withdrawal of growth factors indicating that it is involved in apoptosis. Function Apoptosis Inducing Factor (AIF) is a protein that triggers chromatin condensation and DNA fragmentation in a cell in order to induce programmed cell death. The mitochondrial AIF protein was found to be a caspase-independent death effector that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 12
Caspase 12 is a protein that in humans is encoded by the ''CASP12'' gene. The protein belongs to a family of enzymes called caspases which cleave their Substrate (biochemistry), substrates at C-terminal aspartic acid residues. It is closely related to caspase 1 and other members of the caspase family, known as inflammatory caspases, which process and activate inflammatory cytokines such as interleukin 1 and interleukin 18. Gene It is found on chromosome 11 in humans in a Locus (genetics), locus with other inflammatory caspases. ''CASP12'' orthologs have been identified in numerous mammals for which complete genome data are available. Clinical significance The CASP12 gene is subject to Polymorphism (biology), polymorphism, which can generate a full-length caspase protein (Csp12L) or an inactive truncated form (Csp12S). The functional form appears to be confined to people of Ethnic groups of Africa, African descent and is linked with susceptibility to sepsis; people carrying ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doxorubicin
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein. Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth. Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia. People often experience red discoloration of the urine for a few days. Doxorubicin is in the anthracycline and antitumor antibiotic family of medications. It works in part by interfering with the function of DNA. Doxorubicin was approved for medical use in the United States in 1974. It is on the World Health Organization's List of Essential Medicines. Versions th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diols
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal diol, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
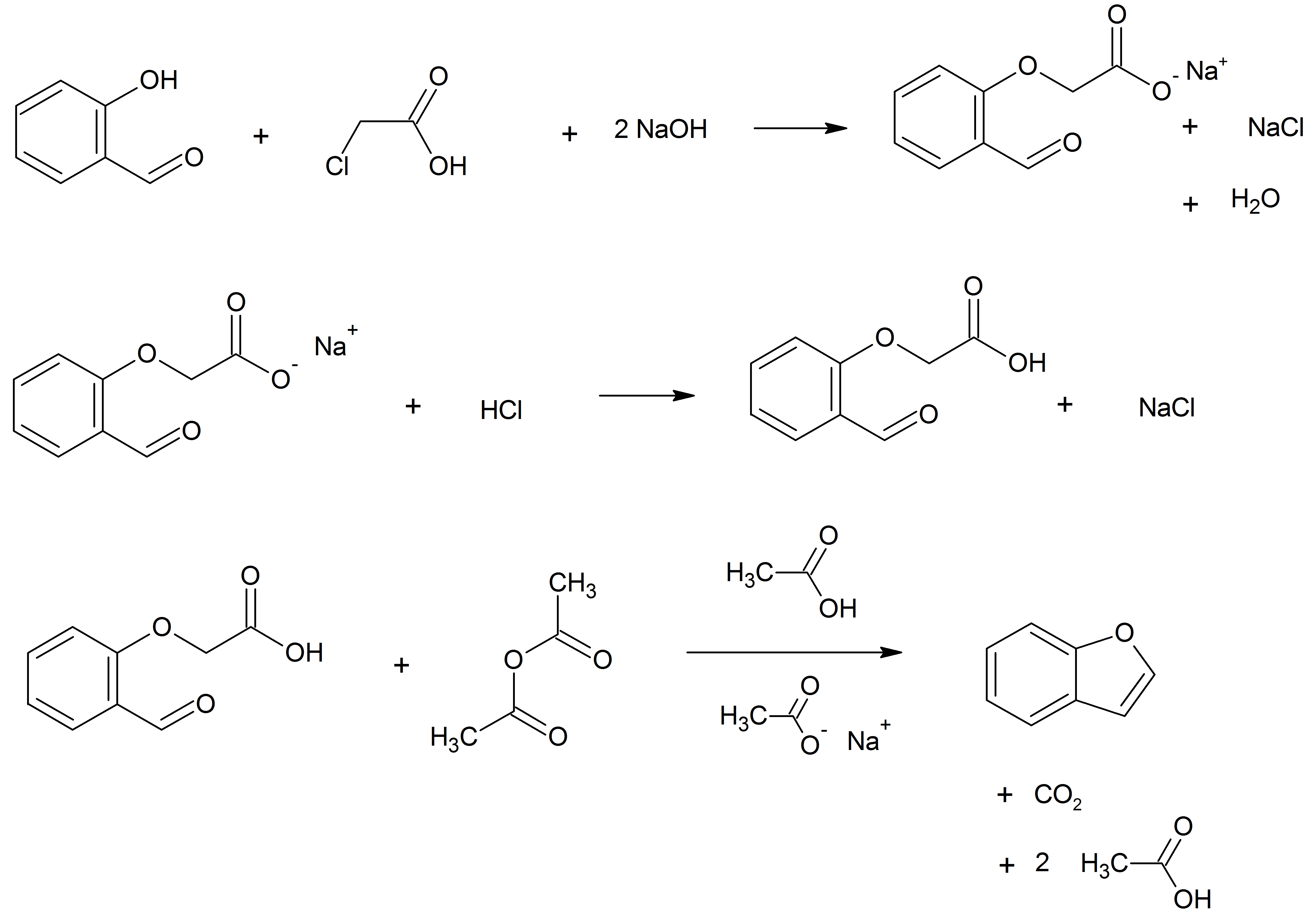
Benzofuran Ethers At The Benzene Ring
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |