|
Frenkel Defect
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the lattice, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion (usually the cation) is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect (does not change the over all stoichiometry of the compound). In ionic compounds, the vacancy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word "crystallography" is derived from the Greek word κρύσταλλος (''krystallos'') "clear ice, rock-crystal", with its meaning extending to all solids with some degree of transparency, and γράφειν (''graphein'') "to write". In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography. denote a direction vector (in real space). * Coordinates in ''angle brackets'' or ''chevrons'' such as <100> denote a ''family'' of directions which are related by symmetry operations. In the cubic crystal system for example, would mean 00 10 01/nowiki> or the negative of any of those directions. * Miller indices in ''parentheses'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Nuclear Charge
In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent higher energy electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. The effective nuclear charge experienced by an electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom. Most of the physical and chemical properties of the elements can be explained on the basis of electronic configuration. Consider the behavior of ionization energies in the periodic table. It is known that the magnitude of ionization potential depends upon the following factors: # Size of atom; # The nuclear charge; # The screening effect of the inner shells, and # The extent to which the outermost electron penetrates into the charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wigner Effect
The Wigner effect (named for its discoverer, Eugene Wigner), also known as the discomposition effect or Wigner's disease, is the displacement of atoms in a solid caused by neutron radiation. Any solid can display the Wigner effect. The effect is of most concern in neutron moderators, such as graphite, intended to reduce the speed of Neutron temperature#Fast, fast neutrons, thereby turning them into Neutron temperature, thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235. Explanation To create the Wigner effect, neutrons that collide with the atoms in a crystal structure must have enough energy to displace them from the lattice. This amount (threshold displacement energy) is approximately 25 Electronvolt, eV. A neutron's energy can vary widely, but it is not uncommon to have energies up to and exceeding 10 MeV (10,000,000 eV) in the centre of a nuclear reactor. A neutron with a significant amount of energy will create a Collision cascade, displa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
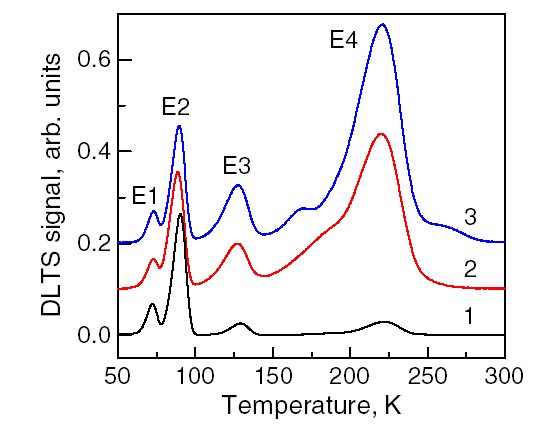
Deep-level Transient Spectroscopy
Deep-level transient spectroscopy (DLTS) is an experimental tool for studying electrically active defects (known as charge carrier traps) in semiconductors. DLTS establishes fundamental defect parameters and measures their concentration in the material. Some of the parameters are considered as defect "finger prints" used for their identifications and analysis. DLTS investigates defects present in a space charge ( depletion) region of a simple electronic device. The most commonly used are Schottky diodes or p-n junctions. In the measurement process the steady-state diode reverse polarization voltage is disturbed by a voltage pulse. This voltage pulse reduces the electric field in the space charge region and allows free carriers from the semiconductor bulk to penetrate this region and recharge the defects causing their non-equilibrium charge state. After the pulse, when the voltage returns to its steady-state value, the defects start to emit trapped carriers due to the thermal emiss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kröger–Vink Notation
Kröger–Vink notation is a set of conventions that are used to describe electric charges and lattice positions of point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by and . Notation The notation follows the scheme: :M *''M'' corresponds to the species. These can be **atoms – e.g., Si, Ni, O, Cl, ** vacancies – V or v (since V is also the symbol for vanadium) ** interstitials – i (although this is usually used to describe lattice site, not species) **electrons – e **electron holes – h *''S'' indicates the lattice site that the species occupies. For instance, Ni might occupy a Cu site. In this case, M would be replaced by Ni and S would be replaced by Cu. The site may also be a lattice interstice, in this case the symbol "i" is used. A cation site can be represented by the symbols C or M (for metal), and an anion site can be represented by either an A or X. *''C'' c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstitial Site
In crystallography, interstitial sites, holes or voids are the empty space that exists between the packing of atoms (spheres) in the crystal structure. The holes are easy to see if you try to pack circles together; no matter how close you get them or how you arrange them, you will have empty space in between. The same is true in a unit cell; no matter how the atoms are arranged, there will be interstitial sites present between the atoms. These sites or holes can be filled with other atoms (interstitial defect). The picture with packed circles is only a 2D representation. In a crystal lattice, the atoms (spheres) would be packed in a 3D arrangement. This results in different shaped interstitial sites depending on the arrangement of the atoms in the lattice. Close packed A close packed unit cell, both face-centered cubic and hexagonal close packed, can form two different shaped holes. Looking at the three green spheres in the hexagonal packing illustration at the top of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(I) Iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is highly photosensitive. This property is exploited in silver-based photography. Silver iodide is also used as an antiseptic and in cloud seeding. Structure The structure adopted by silver iodide is temperature dependent: *Below 420 K, the β phase of AgI, with the wurtzite structure, is most stable. This phase is encountered in nature as the mineral iodargyrite. *Above 420 K, the α phase becomes more stable. This motif is a body-centered cubic structure which has the silver centers distributed randomly between 6 octahedral, 12 tetrahedral and 24 trigonal sites. At this temperature, Ag+ ions can move rapidly through the solid, allowing fast ion conduction. The transition between the β and α forms represents the melting of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(I) Bromide
Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite. Preparation Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide: :AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq) Although less convenient, the compound can also be prepared directly from its elements. Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to produce smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(I) Chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration to some samples. AgCl occurs naturally as a mineral chlorargyrite. Preparation Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis: combining an aqueous solution of silver nitrate (which is soluble) with a soluble chloride salt, such as sodium chloride or cobalt(II) chloride. The silver chloride that forms will precipitate immediately. :AgNO3 + NaCl -> AgCl(v) + NaNO3 :2 AgNO3 + CoCl2 -> 2 AgCl(v) + Co(NO3)2 Structure and reactions The solid adopts the ''fcc'' NaCl structure, in which each Ag+ ion is surrounded by an octahedron of six chloride lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
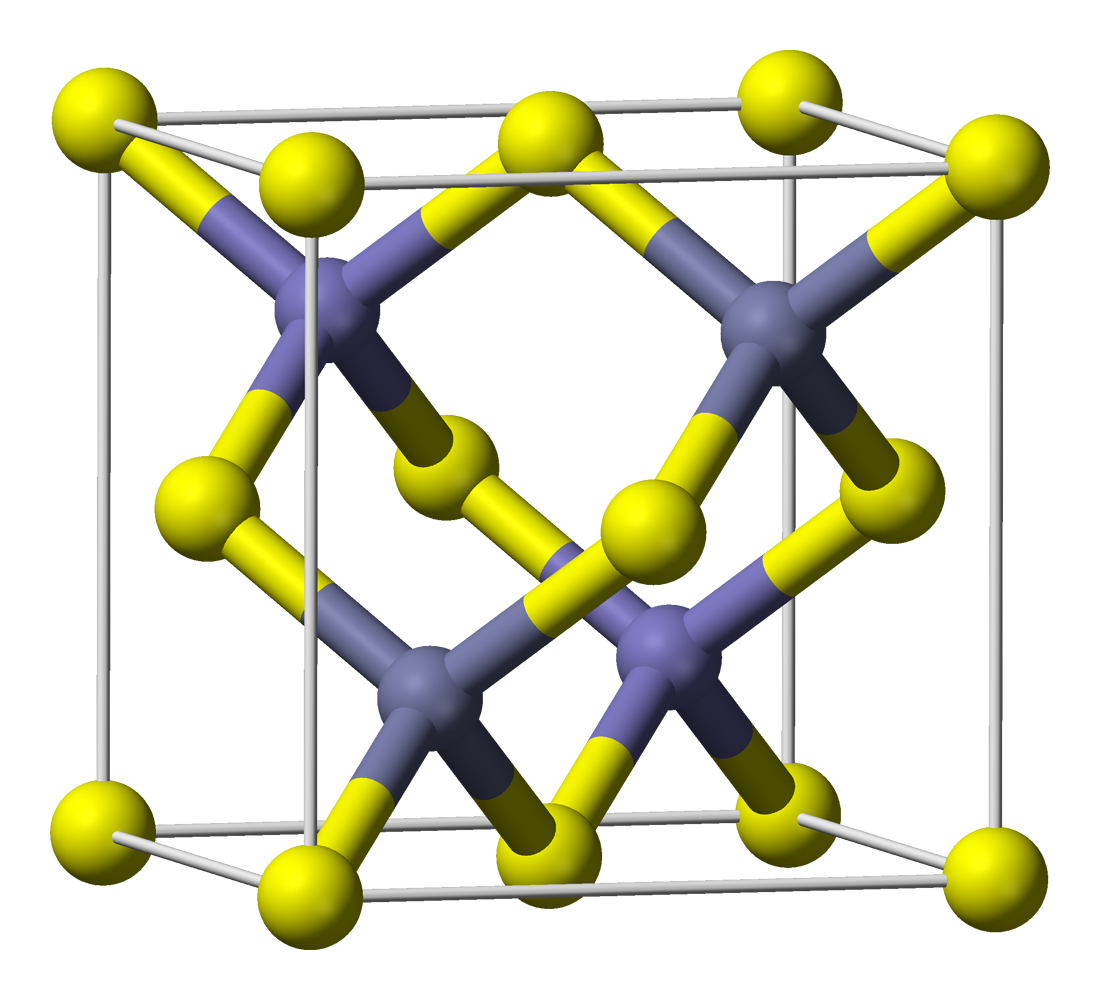
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |