|
Freezing Point Depression Osmometer
The freezing point depression osmometer is an osmometer that is used in determining a solution's osmotic concentration as its osmotically active aspects depress its freezing point. Osmometry further involves other techniques that including membrane osmometry which determines the osmotic pressure of solutions and vapor pressure osmometry which assesses the concentration of particles that minimizes a solution's vapor pressure and melting as well as freezing points of aqueous solutions. Freezing point depression osmometry is, however, the most preferred in distinct contexts. In the past, it has been used to assess the osmotic strength of a colloid and solutions. The osmometer uses the solution's freezing point depression to establish its strength. It is used to determine the level of osmotically appropriate body fluid in various chemicals dissolved in the blood using the relationship which a mole of dissolved substance reduces the freezing point of water by . Being efficient, free ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmometer
An osmometer is a device for measuring the osmotic strength of a solution, colloid, or compound. There are several different techniques employed in osmometry: * Vapor pressure osmometers determine the concentration of osmotically active particles that reduce the vapor pressure of a solution. * Membrane osmometers measure the osmotic pressure of a solution separated from pure solvent by a semipermeable membrane. * Freezing point depression osmometers may also be used to determine the osmotic strength of a solution, as osmotically active compounds depress the freezing point of a solution. Osmometers are useful for determining the total concentration of dissolved salts and sugars in blood or urine samples. Osmometry is also useful in determining the molecular weight of unknown compounds and polymers. Osmometry is the measurement of the osmotic strength of a substance. This is often used by chemists for the determination of average molecular weight A molecule is a group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometer
330px, Different lengths as in respect to the molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, -er, American spelling) is a units of measurement, unit of length in the International System of Units (SI), equal to one billionth (short scale) of a metre () and to 1000 picometres. One nanometre can be expressed in scientific notation as , and as metres. History The nanometre was formerly known as the millimicrometre – or, more commonly, the millimicron for short – since it is of a micron (micrometre), and was often denoted by the symbol mμ or (more rarely and confusingly, since it logically should refer to a ''millionth'' of a micron) as μμ. Etymology The name combines the SI prefix ''nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colligative Properties
In chemistry, colligative properties are those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the nature of the chemical species present. The number ratio can be related to the various units for concentration of a solution such as molarity, molality, normality (chemistry), etc. The assumption that solution properties are independent of nature of solute particles is exact only for ideal solutions, which are solutions that exhibit thermodynamic properties analogous to those of an ideal gas, and is approximate for dilute real solutions. In other words, colligative properties are a set of solution properties that can be reasonably approximated by the assumption that the solution is ideal. Only properties which result from the dissolution of a nonvolatile solute in a volatile liquid solvent are considered.KL Kapoor ''Applications of Thermodynamics'' Volume 3 They are essentially solvent pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling-point Elevation
Boiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The boiling point can be measured accurately using an ebullioscope. Explanation The ''boiling point elevation'' is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. It is an effect of the dilution of the solvent in the presence of a solute. It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solute–solvent interactions. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. In thermodynamic terms, the origin of the boiling point elevation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting-point Depression
:''This article deals with melting/freezing point depression due to very small particle size. For depression due to the mixture of another compound, see freezing-point depression.'' Melting-point depression is the phenomenon of reduction of the melting point of a material with reduction of its size. This phenomenon is very prominent in nanoscale materials, which melt at temperatures hundreds of degrees lower than bulk materials. Introduction The melting temperature of a bulk material is not dependent on its size. However, as the dimensions of a material decrease towards the atomic scale, the melting temperature scales with the material dimensions. The decrease in melting temperature can be on the order of tens to hundreds of degrees for metals with nanometer dimensions. Melting-point depression is most evident in nanowires, nanotubes and nanoparticles, which all melt at lower temperatures than bulk amounts of the same material. Changes in melting point occur because nanoscale m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolality
Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent. This contrasts with the definition of molarity which is based on a specified volume of solution. A commonly used unit for molality in chemistry is mol/ kg. A solution of concentration 1 mol/kg is also sometimes denoted as ''1 molal''. The unit ''mol/kg'' requires that molar mass be expressed in ''kg/mol'', instead of the usual ''g/mol'' or ''kg/kmol''. Definition The molality (''b''), of a solution is defined as the amount of substance (in moles) of solute, ''n''solute, divided by the mass (in kg) of the solvent, ''m''solvent: :b = \frac In the case of solutions with more than one solvent, molality can be defined for the mixed solvent considered as a pure pseudo-solvent. Instead of mole solute per kilogram solvent as in the binary case, units are defined as mole solute per kilogram mixed solvent. Origin The term ''molality'' is formed in analogy to ''mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
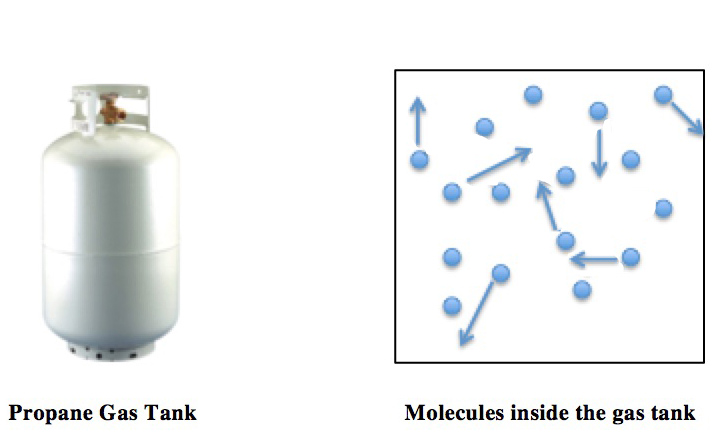
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfred Nobel was a Swedish chemist, engineer, and industrialist most famously known for the invention of dynamite. He died in 1896. In his will, he bequeathed all of his "remaining realisable assets" to be used to establish five prizes which became known as "Nobel Prizes." Nobel Prizes were first awarded in 1901. Nobel Prizes are awarded in the fields of Physics, Chemistry, Physiology or Medicine, Literature, and Peace (Nobel characterized the Peace Prize as "to the person who has done the most or best to advance fellowship among nations, the abolition or reduction of standing armies, and the establishment and promotion of peace congresses"). In 1968, Sveriges Riksbank (Sweden's central bank) funded the establishment of the Prize in Economi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Litre
The litre (international spelling) or liter (American English spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metre (m3). A cubic decimetre (or litre) occupies a volume of (see figure) and is thus equal to one-thousandth of a cubic metre. The original French metric system used the litre as a base unit. The word ''litre'' is derived from an older French unit, the '' litron'', whose name came from Byzantine Greek—where it was a unit of weight, not volume—via Late Medieval Latin, and which equalled approximately 0.831 litres. The litre was also used in several subsequent versions of the metric system and is accepted for use with the SI,Bureau International des Poids et M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Concentration
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of ''osmoles of solute particles'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the osmole. This is a non- SI unit of measurement that defines the number of moles of solute that contribute to the osmotic pressure of a solution. A milliosmole (mOsm) is 1/1,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Chemistry
Clinical chemistry (also known as chemical pathology, clinical biochemistry or medical biochemistry) is the area of chemistry that is generally concerned with analysis of bodily fluids for diagnostic and therapeutic purposes. It is an applied form of biochemistry (not to be confused with medicinal chemistry, which involves basic research for drug development). The discipline originated in the late 19th century with the use of simple chemical reaction tests for various components of blood and urine. In the many decades since, other techniques have been applied as science and technology have advanced, including the use and measurement of enzyme activities, spectrophotometry, electrophoresis, and immunoassay. There are now many blood tests and clinical urine tests with extensive diagnostic capabilities. Most current laboratories are now highly automated to accommodate the high workload typical of a hospital laboratory. Tests performed are closely monitored and quality controll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |