|
Foldamer
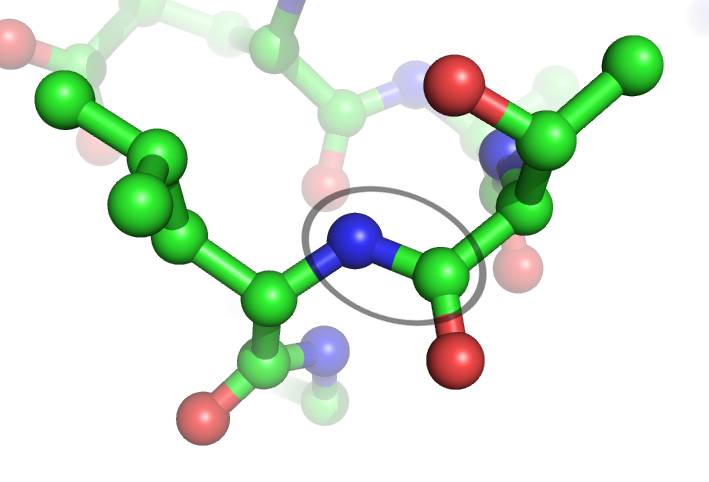
In chemistry, a foldamer is a discrete chain molecule (oligomer) that folds into a conformationally ordered state in solution. They are artificial molecules that mimic the ability of proteins, nucleic acids, and polysaccharides to fold into well-defined conformations, such as α-helices and β-sheets. The structure of a foldamer is stabilized by noncovalent interactions between nonadjacent monomers. Foldamers are studied with the main goal of designing large molecules with predictable structures. The study of foldamers is related to the themes of molecular self-assembly, molecular recognition, and host–guest chemistry. Design Foldamers can vary in size, but they are defined by the presence of noncovalent, nonadjacent interactions. This definition excludes molecules like poly(isocyanates) (commonly known as (polyurethane)) and poly(prolines) as they fold into helices reliably due to ''adjacent'' covalent interactions., Foldamers have a dynamic folding reaction nfolded â ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folding (chemistry)
In chemistry, folding is the process by which a molecule assumes its shape or conformation. The process can also be described as intramolecular self-assembly, a type of molecular self-assembly, where the molecule is directed to form a specific shape through noncovalent interactions, such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi-pi interactions, and/or electrostatic effects. The most active area of interest in the folding of molecules is the process of protein folding, which is the shape that is assumed by a specific sequence of amino acids in a protein. The shape of the folded protein can be used to understand its function and design drugs to influence the processes that it is involved in. There is also a great deal of interest in the construction of artificial folding molecules or foldamers. They are studied as models of biological molecules and potential application to the development of new functional materials. See also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lehn Beautiful Foldamer HelvChimActa 1598 2003 (born 1977), Norwegian football midfielder
{{surname ...
Lehn is a surname. Notable people with this surname include: * Abraham Lehn (landowner) (1702–1757), Danish landowner * Christian vom Lehn (born 1992), German swimmer * Erwin Lehn (1919–2010), German jazz musician * Jean-Marie Lehn (born 1939), French chemist * Poul Abraham Lehn, Danish nobleman * Thomas Lehn (born 1958), German musician * Unni Lehn Unni Lehn (born 7 June 1977) is a retired Norwegian football midfielder. She has 133 appearances for Norway's national team. In 2000 Lehn played 86 minutes of the Olympic Final in Sydney, in which Norway beat the US in extra time to take the go ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folding Energy Diagram
Fold, folding or foldable may refer to: Arts, entertainment, and media * ''Fold'' (album), the debut release by Australian rock band Epicure *Fold (poker), in the game of poker, to discard one's hand and forfeit interest in the current pot *Above the fold and below the fold, the positioning of news items on a newspaper's front page according to perceived importance *Paper folding, or ''origami'', the art of folding paper Science, technology, and mathematics Biology *Protein folding, the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure **Folding@home, a powerful distributed-computing project for simulating protein folding *Fold coverage, quality of a DNA sequence *Skin fold, an area of skin that folds Computing *Fold (higher-order function), a type of programming operation on data structures *fold (Unix), a computer program used to wrap lines to fit in a specified width *Folding (DSP implementation), a transformation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a ''dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular Forces
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic forc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Collapse
Hydrophobic collapse is a proposed process for the production of the Protein structure, 3-D conformation adopted by Peptide, polypeptides and other molecules in polar solvents. The theory states that the nascent polypeptide forms initial Protein secondary structure, secondary structure (Alpha helix, ɑ-helices and Beta sheet, β-strands) creating localized regions of predominantly hydrophobic Amino acid, residues. The polypeptide interacts with water, thus placing Hydrophobic effect, thermodynamic pressures on these regions which then aggregate or "collapse" into a Protein tertiary structure, tertiary conformation with a hydrophobic core. Incidentally, polar residues interact favourably with water, thus the solvent-facing surface of the peptide is usually composed of predominantly Hydrophile, hydrophilic regions. Hydrophobic collapse may also reduce the affinity of conformationally flexible drugs to their protein targets by reducing the net hydrophobic contribution to binding by se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre (N/m2); similarly, the pound-force per square inch (psi) is the traditional unit of pressure in the imperial and U.S. customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as of this. Manometric u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Root Mean Square
In mathematics and its applications, the root mean square of a set of numbers x_i (abbreviated as RMS, or rms and denoted in formulas as either x_\mathrm or \mathrm_x) is defined as the square root of the mean square (the arithmetic mean of the squares) of the set. The RMS is also known as the quadratic mean (denoted M_2) and is a particular case of the generalized mean. The RMS of a continuously varying function (denoted f_\mathrm) can be defined in terms of an integral of the squares of the instantaneous values during a cycle. For alternating electric current, RMS is equal to the value of the constant direct current that would produce the same power dissipation in a resistive load. In estimation theory, the root-mean-square deviation of an estimator is a measure of the imperfection of the fit of the estimator to the data. Definition The RMS value of a set of values (or a continuous-time waveform) is the square root of the arithmetic mean of the squares of the values, or th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |